Background and Signalling Pathway
From 2006.igem.org
| Home | Project | Methods | Results & Conclusions | Terms & References | Team members |
|---|
Background
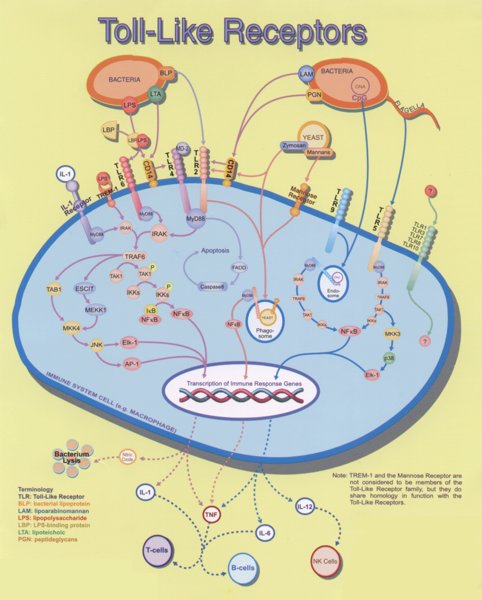
When bacteria invade the human body, it has to respond quickly by activation of the innate branch of the immune system, which recognizes the broad spectrum of molecules, specific for pathogenic microorganisms. Immune response cells have on their surface a collection of receptors (Toll-like receptors - TLR), which recognize those "Pathogen associated molecular patterns" or PAMPs. PAMPs include various components of microorganisms (e.g. lipopolysaccharides, which are part of outer cell membrane of Gram-negative bacteria, flagellin, lipopeptides but also double-stranded RNA from viruses etc., which are each recognized by one of the 11 human TLRs). This kind of cell signalling is the core of our efficient innate immune response (i.e. the fast-acting, not mediated by antibodies) against microbes. In some instances, however, extensive signaling mediated by TLR is not beneficial. If the cellular response gets out ouf control and becomes exaggerated, excessive inflammatory response may lead to sepsis, which can lead to severe organ failure and often the consequences are fatal. Sepsis claims each year more than 150,000 casualties within the USA and a similar number within the EU. In the absence of an effective treatment of sepsis, the analysis of the signaling pathway may indicate the potential therapeutic targets.
Response to bacterial stimulus is a double-edged sword - too strong response may lead to sepsis, while the too weak response does not overcome the bacterial infection, therefore both extremes have to be in the balance. The feedback mechanism that would limit the excessive cell stimulation, but would nevertheless maintain the responsiveness to bacterial infection could provide the path to an effective antiseptic strategy.
Signaling pathway of bacterial recognition in a nutshell
Toll-like receptors (TLRs) are the key gatekeepers of innate immunity, sensing and responding to invading microorganisms. So far in humans eleven different receptors are konwn that recognize specific molecular patterns which are specific for microbes, e.g. lipopolysaccharide (LPS), lipopeptides, double and single stranded RNA of viruses, nonmethylated CpG containing DNA, glycolipids, and several others.
Most of the receptors and transducers in the signaling cascade involved in the initial stages of cell transduction have been identified, however the complete response is highly complex as for example LPS alone can affect transcription of more than 1000 different genes.
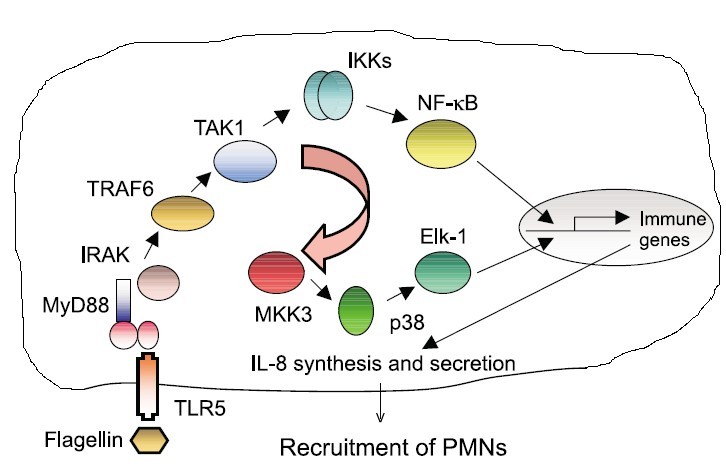
Binding of bacterial constituents (PAMPs) to TLR triggers the association of its intracellular TIR domains, which recruit from the cytoplasm to the membrane an adapter molecule MyD88, which in turn transmits the signal to other components of the signaling cascade, particularly kinases, as shown in Figure 2.
When receptors sense the presence of microbial components, stimulation of TLRs occurs and triggers the association of MyD88 (myeloid differentiation primary-response protein 88), which in turn recruits IRAK4, thereby allowing its association with IRAK1. IRAK4 phosphorylates IRAK1 and with phosphorylated IRAK1 associates TRAF6 (tumour necrosis factor receptor- associated factor 6). The complex of phosphorylated IRAK1 and TRAF6 dissociates from the receptor and forms a complex with TAK1, TAB1 and TAB2, which induces the phosphorylation of TAB2 and TAK1. This leads to the ubiquitylation of TRAF6, which induces the activation of TAK1. TAK1 phosphorylates IKK complex (inhibitor of nuclear factor κB (IκB)-kinase complex), which consists of IKKα, IKKβ and IKKγ. The IKK complex then phosphorylates IκB, which is associated with NF-κB thus enabling its translocation to the nucleus. Phosphorylation of IκB leads to its ubiquitylation and subsequent degradation. This allows the important inflammatory transcription factor NF-κB to release and to translocate to the nucleus and to induce the transcription of a range of its target genes, such as cytokines and chemokines.
Inflammatory cytokines, chemokines and interferons attract cells of the immune system (lymphocytes, macrophages) to the infected area. This is beneficial to organism, but if overactivated it can also lead to severe inflammation and sepsis.
| Home | Project | Methods | Results & Conclusions | Terms & References | Team members |
|---|