Construction
From 2006.igem.org
< Aug11-Sep7 | Sep8-14 >
Contents |
September 27, 2006
Charles:
- made freezer stocks of UT2 x 2 and UT3 x 2
Andy, Natalie:
- Minipreppred UT3 B(AB) C(BB) D(BC) E(CC)
- transformed and plated P0452 (2005) (On Amp rather than proper AK)
- checked lengths of UT3 BCDE, P0152, P0352
- Prepared o/n of P0456 (2005) for Miniprep
To-Do List:
- Miniprep P0456 (2005)
- Take P0456, P0452, P0152, P0352 from 2006 registry
- Make more plates, make more LB
- Learn to use new DH5a (with no LacI expression) for testing
- Post up new protocols, include part length and resistance info in spreadsheet
September 26, 2006
Charles:
- Prepared o/n of UT2 (2 vials) and UT3 (2 vials) for a freezer stock
Andy:
- Prepared o/n of UT3 BCDE for mini-preps
- transformed and plated P0452 (2005) and P0456 (2005)
To-Do List:
- Make freezer stock and minipreps of above tubes and check lengths along with P0352 (2005) and P0152 (2005)
- Make more plates, autoclave tips, make more LB
- Post up new protocols, include part length and resistance info in spreadsheet
September 22, 2006
To-Do List:
- Make frozen stock of UT2 and UT3
- Prepare o/n for UT3 BCDE for mini-prep
- Post up new protocols, include part length and resistance info in spreadsheet
- Make more plates and re-plate P0452 (2005) – AK, and P0456 (2005) – AC
September 21, 2006
Charles:
- Made 22 competent cells
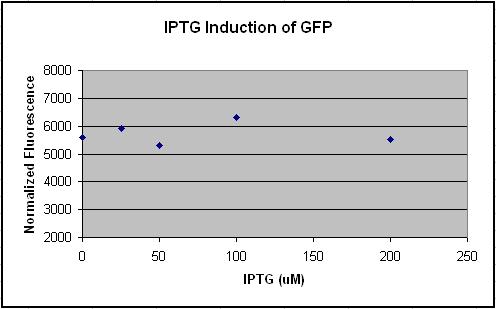
- Tested Module 1 using varying concentrations of IPTG (0, 2, 20, 200, 2000 uM) to induce GFP
(E0240 (2005)) and mRFP (I13507 (2005))
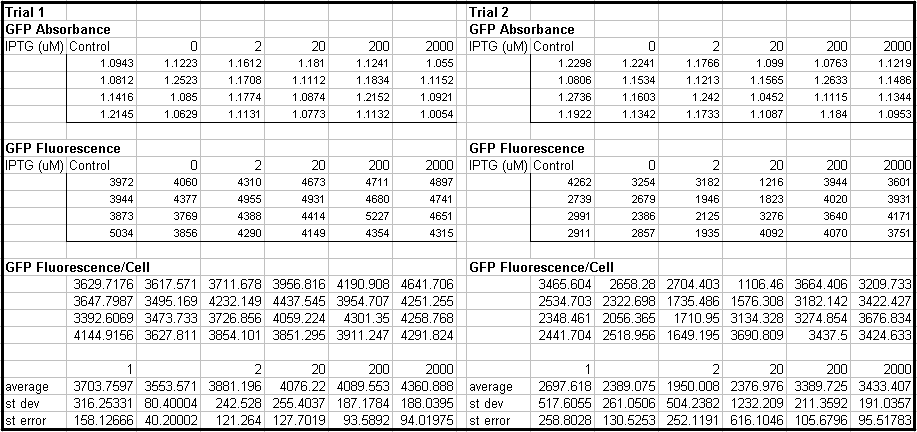
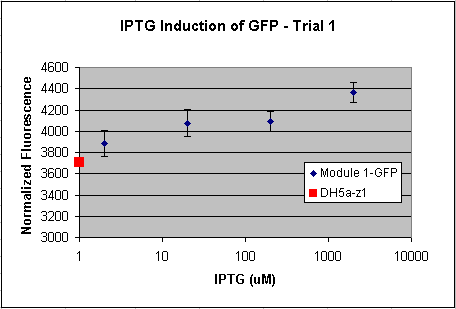
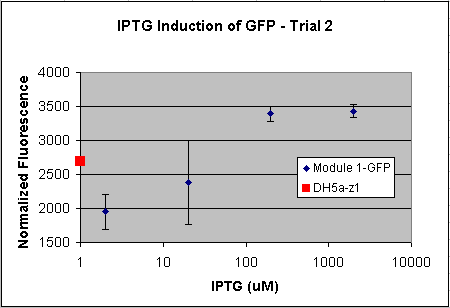
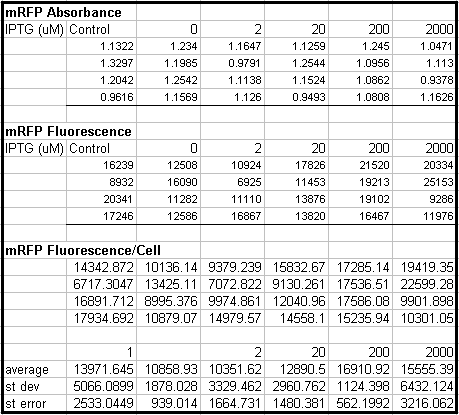
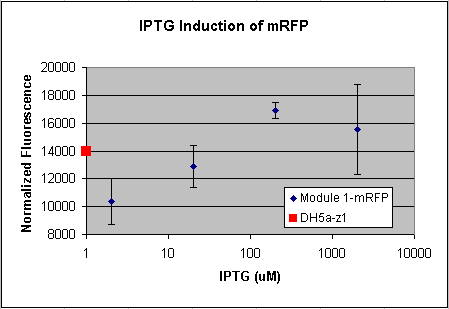
- Data indicates that the GFP and mRFP as well as the LacI+ pL promoter (R0011) are functional (Does not indicate functionality of the pBad/AraC promoter and the inverter)
- Some of the replicates seem to be outliers and if excluded, the regression increases significantly
- DH5a-z1 are known to fluoresce, so the fact that untransformed cells glow brighter is not a big concern
- Next step is to transform UT2 and UT3 into LacI negative cells then:
- Characterize the fluorescence under varying Arabinose concentrations.
- Vary the temperature to verify the temperature sensitivity of LacI ts and its affects on reporter expression
</ul>
To-Do List:
- Post up new protocols, include part length and resistance info in spreadsheet
- Make more plates and re-plate P0452 (2005) – AK, and P0456 (2005) – AC
September 20, 2006
Natalie, Andy:
- Prepared o/n growth of UT3 BCDE for mini-prep, DH5a for competent cells, and UT2 – 3,4,5, UT3 – 7,8,9 and DH5a x 2 (control) for making fluorescence measurements.
- Checked lengths of miniprep UT2 ABCDEF, UT3 A (possibly unreliable due to 2 day o/n growth), P0352 (2005), and P0152 (2005).
To-Do List:
- Make 24 competent cells
- Miniprep UT3 BCDE and test lengths
- Make more plates and re-plate P0452 (2005) – AK, and P0456 (2005) – AC
- Do measurements on UT2 and UT3
- Post up new protocols, include part length and resistance info in spreadsheet
September 18, 2006
Charles:
- Only mini-prepped UT3 A because they were grown for two o/n (Just want to see what happens)
- Prepared new o/n for UT3 BCDE as well as 1 DH5a-z1, 2 UT2 and 2 UT3 for testing tomorrow. And 1 DH5a-z1 for making competent cells
To-Do List:
- Make 24 competent cells
- Test the lengths of UT2 ABCEDF, P0352 (2005), P0152 (2005), and UT3 BCDE
- Make more plates and re-plate P0452 (2005) – AK, and P0456 (2005) – AC
- Prepare new o/n for UT3 BCDE – for mini-prep
- Prepare o/n in the following quantities: 3 DH5a-z1, 3 UT2 and 3 UT3
September 16, 2006
Charles:
- Made 4 replications of UT2 in 200 uM, 100 uM, 50 uM, 25 uM and 0 uM of IPTG.
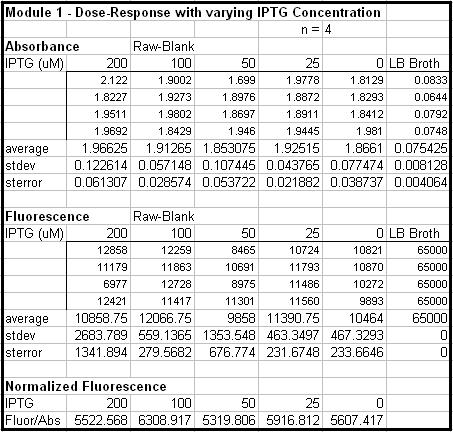
- OD of cells is too high, need to be <= 1.2
- Need a control of untransformed DH5a-z1 to compare the relative fluorescence
- Made frozen stock (2 vials each): UT01, I13507 (2005), B0015 (2005), B0031 (2005)
- Mini-prepped UT2 ABCEDF, P0352 (2005) and P0152 (2005)
To-Do List:
- Test the lengths of UT2 ABCEDF, P0352 (2005) and P0152 (2005)
- Prepare an o/n growth of the “best” UT2 for testing
- Prepare an o/n growth of regular DH5a-z1
- Mini-prep UT03 ABCD and test their lengths along with P00352 (2005) and P0152 (2005)
- Re-plate P0452 (2005) - AK, and P0456 (2005) - AC
- Make 24 competent cells
September 15, 2006
Andy, Charles, Natalie, Stan, Elliott:
- Checked and transformed I+J+I plasmids (Bands: ~6000-5000, 3500-2500 (smear))
- Made competent cells (although, may have to repeat)
- Prepared o/n growths of I+J+E (12 vials), P0152 (2005) and P0352 (2005). P0456 (2005) and P0452 (2005) did not seem to grow very well so left them in the incubator for a 2nd night
- Made 8 Amp plates
To-Do List:
- Mini-prep P0152 (2005), P0352 (2005) and 6 of the I+J+E
- Try to get J04450 (2006) working again.
- Check lengths of R0011 (2005/6), if no good, then obtain R0010 (2005/6) from Registry
- Make freezer stock (2 each) of I+J, B0015 (2005), B0031 (2005), and I13507 (2005) and put in -80C freezer stock
- Test the fluorescence of I+J+E