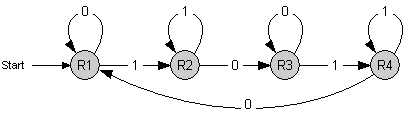ETH Zurich 2006
From 2006.igem.org
(→Conclusion and Outlook) |
|||
| Line 158: | Line 158: | ||
By exploring the parameter space and performing [[Modeling_and_overall_design#Sensitivity_Analysis|Sensitivity Analysis]], the following conclusions could be drawn: | By exploring the parameter space and performing [[Modeling_and_overall_design#Sensitivity_Analysis|Sensitivity Analysis]], the following conclusions could be drawn: | ||
| - | * Changes in the affinity/cooperativity of the | + | * Changes in the affinity/cooperativity of the input signal S affect the system more strongly than changes in the affinity/cooperativity of the zincfingers. |
| - | * The degradation rate of the | + | * The affinities of the zincfingers/input signal S are more sensitive than the cooperativities. |
| + | * The maximum expression rate and the degradation rate of the zincfingers are the most sensitive parameters. The maximum expression rate is a bit more sensitive, but they are quite close together. | ||
* The affinity constants R1..R4 should be as symmetrical as possible, in particular the couples R1,R3 and R2,R4. A difference in the affinity constant up to an order of magnitude appears tolerable. | * The affinity constants R1..R4 should be as symmetrical as possible, in particular the couples R1,R3 and R2,R4. A difference in the affinity constant up to an order of magnitude appears tolerable. | ||
* ... | * ... | ||
Revision as of 21:42, 31 October 2005
Contents |
News
- 2005.10.25 Now we know that Blue Heron will not be able to deliver in time.
- 2005.10.24 We had a very intensive meeting on the overall concept and the documentation of the project. This page will change a lot in the coming days.
- 2005.10.23 One month after ordering, the sequences are still not ready. That means it is unlikely for us to complete the 4-State Device before the jamboree unless we find some alternative solutions (and have quite a bit of luck). See message from blue herons web page:
IMPORTANT NOTE: Despite Blue Heron Bio's capacity expansion, record order volume has created larger than anticipated queuing, which has increased delivery times for some orders. We are reviewing Estimated Ship Dates regularly to provide you with our best estimate of when your order will ship. We are working hard to deliver your orders as quickly as possible, and we are implementing new capacity management systems to avoid this problem in the future. If you have any questions or concerns please call our Customer Service.
- 2005.10.18 The parts for the actual Event Processing Device are ready, thanks to the hard work of Giorgia, Hervé, and Martje (not all test/debugging parts though)
- 2005.10.07 Message from Blue Heron: Sequences for NOR are synthesized and will be verified and assembled next week.
- 2005.09.23 Sequences for 4-State Device ordered from Blue Heron
Organisation
People
Students
| Simon Barkow | Christophe Dessimoz | Zlatko Franjcic |
| Dominic Frutiger | Robin Künzler | Urs A. Müller |
| Jonas Nart | Kristian Nolde | Alexander Roth |
| Tamara Ulrich | Giorgia Valsesia | Herve Vanderschuren |
Supervisors
| Jörg Stelling | Sven Panke | Eckart Zitzler |
Advisors
| Uwe Sauer | Martin Fussenegger | Andreas Hierlemann |
| Kay-Uwe Kirstein | Ruedi Aebersold |
Timeline
Tasks
=Abstract= The project of the ETH Zurich team consists of the design and implementation in vivo of a gene circuit that can count to 2. In essence, the counter uses two toggle switches, each storing 1 bit, to keep track of the 4 internal states. The design of the counter is highly modular, with the hope that it can be included as a unit in larger circuits, and also combined with further counter instances to keep track of a much larger number of states, up to 2^n with n units. To facilitate further developments and integration to other projects, the counter is available in form of BioBricks. Among many exciting applications, the availability of a counter enables the execution of sequential instructions, and paves the way for the execution of artificial programs inside living cells.
Introduction
The past few years have seen the emergence of the field of Synthetic Biology, in which functional units are designed and built into living cells to generate a particular behaviour, and ultimately to better understand Life's mechanisms. Previous efforts include the creation of gene circuits that generate oscillating behaviour (Elowitz00), toggle switch functionality (Atkinson03), artificial cell-cell communication (Bulter04) or pattern-forming behaviour (Basu2005). The present document describes the design and realization of a gene circuit as a basic device that counts external events to 2.
Concept
The counter is a finite state machine implemented as a genetic circuit. It has 4 internal states s1 to s4. The transition between these states is induced by an external stimulus with values 0 and 1 - denoting whether it is absent or present, respectively. Repeated stimulus will lead to successive transitions and finally to repeated cycling through those 4 states.
Each time the state s3 is reached, an output signal is generated. This leads to a counting behavior where every second occurence of 1 (high signal) is indicated by the output signal.
Further information on the state machine
The coupling of further instances of this state machine allows to count to higher numbers, up to 2^n with n units.
System Implementation
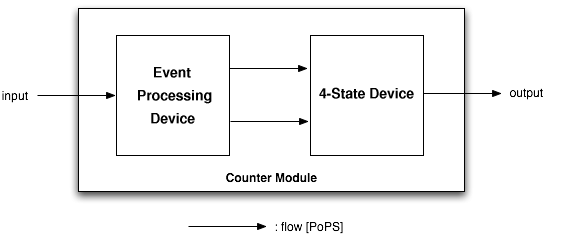
System Architecture
System-level Diagram
Device-level Diagram
As depicted above, the counter is made of two parts, serially linked:
- The Event Processing Device, which splits the input into two outputs: one feed through and one inverted signal - which serve in turn as inputs for the 4-State Device.
- The 4-State Device module, which uses these two signals to sequentially switch through the states S1, S2, S3 and S4.
Note that all interfaces have flows described in Polymerase Per Second (PoPS), is explained in details on the abstraction hierarchy of the MIT Registry of Parts. For instance, the input can be of any nature as long as an adequate promoter is available (e.g. heat-shock using a sigma32 promoter, IPTG using a LacI promoter, AHL using quorum sensing promoters...)
Parts-level Diagram
... parts with the typical illustration as found registry.
Concentration Dynamics Diagram
Devices
Event Processing Device
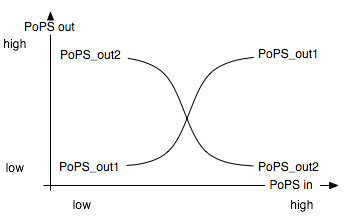
Basic Functionality
The Event Processing Device splits the input induced by an external event into two signals: one is basically fed through while the other is inverted. It is best described through its system boundaries. One of the outputs should be high and the other low when S is high and vice versa when S is low [Fig EPD.1]. Both output signals then serve as input signals for the 4-State Device and it is thus necessary that they have the same delay.
Biological Implementation
Biologically, a NOR gate can be implemented by using a promoter with high basal activity that is repressed by three effectors. If there is at least one of the three repressors, transcription is inhibited. After each of the four NOR gates, there is a coding region for a protein.
To connect the output of such a gate (A) to the input of an other one (B), we just need to ensure that gate A produces a repressor protein for which there is a corresponding binding site at the input of gate B.
We use Zinc Finger proteins (ZFP) as repressors. This class of proteins binds to specific base pairs on the DNA. Many protein-DNA interaction for ZF domains and triplet of base pairs have been described, therefore making it possible to construct artificial transcription factors by combining ZF domains in a modular fashion. The idea is to use a ZFP as a repressor by putting a binding site for a ZFP upstream of the coding region and thereby preventing RNA polymerase to transcribe the gene.
Detailed Documentation
For more details, please consult the main page for the Event Processing Device.
4-State Device
Basic Functionality
The 4-State Device uses two inputs to sequentially switch through four states. At every second occurence of a high input signal, the third state is reached. Note that this behaviour can be observed abstractly in the concept section.
To implement this behaviour, we use the idea of an electronic circuit with four NOR gates, each having three input signals. These gates are connected in such a way, that at any time one of them has high and all the others have low output. We define the gate with high output as the active state. Each time the main input signal rises or drops, the active state ist changed: The next gate in the sequence becomes active.
Biological Implementation
We use Zinc Finger proteins (ZFP) as repressors. This class of proteins binds to specific base pairs sequence on the DNA. Many protein-DNA interactions for ZF domains and triplet of base pairs have been described, therefore making it possible to design artificial transcription factors by combining ZF domains in a modular fashion. The idea is to use a ZFP as a repressor by putting a binding site for a ZFP upstream of the coding DNA sequence and thereby preventing RNA polymerase from transcribing this coding sequence.
Detailed Documentation
More details can be found in the dedicated 4-State Device page.
Mathematical Modeling
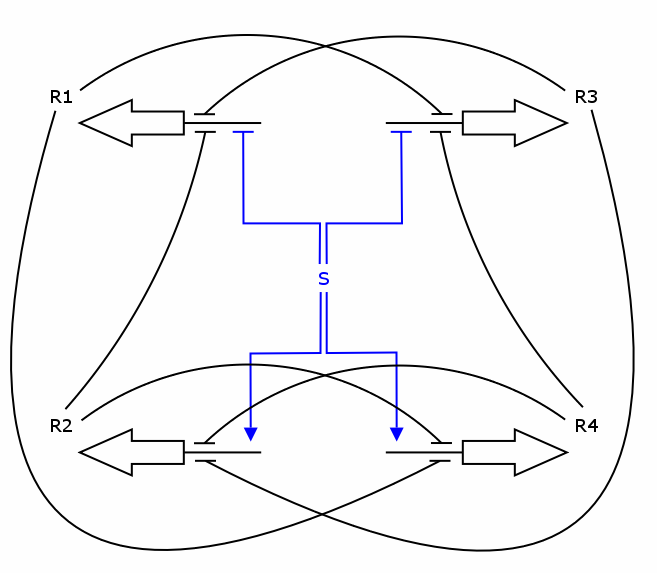
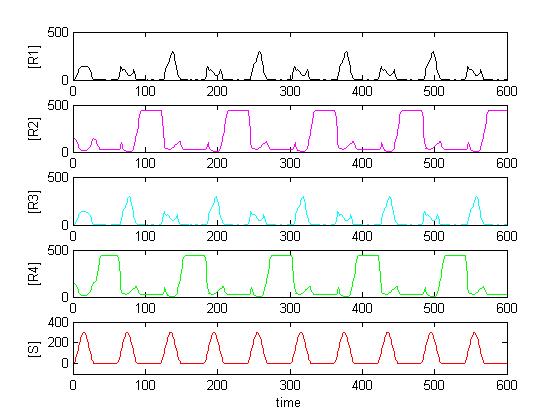
The simulation was performed through a deterministic model using ordinary differential equations (ODEs), as this approach is commonly used in modeling gene networks. Recall the counter architecture in [Figure Simulation 1].
R1 to R4 are the zincfingers that are expressed. S is the input of the counter. Note that the work of the Event Processing Device is symbolized by the dual effect of S, once as an activator and once as a repressor. Zincfingers R1 and R3 have Pr as a promoter, R2 and R4 have Prm as a promoter. See Event_Processing_Device for more details.
Note: This figure needs to be updated!
The 4 corresponding differential equations are:
dR1/dt = k_syn_R1 * rep(S) * rep(R2) * rep(R3) - k_deg_R1 * R1
dR2/dt = k_syn_R2 * act(S) * rep(R3) * rep(R4) - k_deg_R2 * R2
dR3/dt = k_syn_R3 * rep(S) * rep(R1) * rep(R4) - k_deg_R3 * R3
dR4/dt = k_syn_R4 * act(S) * rep(R1) * rep(R2) - k_deg_R4 * R4
\_________________ _________________/ \_______ _______/
V V
synthesis rate degradation rate
where act(A) = (A/K_act)^n / (1+(A/K_act)^n)
and rep(R) = 1 / (1+(R/K_rep)^n)
K are affinity constants, while k are kinetic constants.
Solving the system in Matlab using reasonable affinity and kinetic constants, the result can look as in [Figure Simulation 2].
As expected, [R4] follows every second peak of [S].
By exploring the parameter space and performing Sensitivity Analysis, the following conclusions could be drawn:
- Changes in the affinity/cooperativity of the input signal S affect the system more strongly than changes in the affinity/cooperativity of the zincfingers.
- The affinities of the zincfingers/input signal S are more sensitive than the cooperativities.
- The maximum expression rate and the degradation rate of the zincfingers are the most sensitive parameters. The maximum expression rate is a bit more sensitive, but they are quite close together.
- The affinity constants R1..R4 should be as symmetrical as possible, in particular the couples R1,R3 and R2,R4. A difference in the affinity constant up to an order of magnitude appears tolerable.
- ...
More details of the simulation work are reported on the page Mathematical_Modeling.
Results
We have ongoing experiments and the documentation is not up to date / well structured. In the meantime, please refer to the Experiments page.
Discussion
Outlook
As already mentioned in the introduction the field of Synthetic Biology is still very young but shows a lot of potential. At this stage every new development can have huge effects on the general focusing of the field. During our project we came to the point where we had to decide whether we want to go for a cool and fancy behaviour or for a part that can be reused in the future for many other things (see the previous ideas). The result described in detail in the above sections show obviously that we went for the ladder. People who hear about Synthetic Biology for the first time by reading about our project might think: “Why should a bacterium be able to count to two?” A good question. In order to give an answer and also a feeling about the mightiness of our approach to the reader, we want to list a few of our ideas in the following:
One of the first things that comes to one’s mind is to add further 4SDs in series. This way you could not only count to 2 but to 2^n, where n is the number of 4SDs. You only have to change the zincfingerproteins of each 4SD in order to avoid interactions (for 3 fingers there are already about 250’000 possible combinations). With 64 of these 4SDs we would already have the instruction length of the latest computers.
But our counter can’t only be seen in the common sense. You can also use it to provide a certain sequence of actions. By adding to carefully selected states an extra DNA strand coding a protein one could control a complicated sequence by only giving a input signal at the event processing device. The network of 4SDs would do the rest. The network could also have parallel parts as the output of one state could be used as input of two or more other 4SDs.
As in the Registry of Standard Biological Parts the inputs and outputs of the parts are defined by PoPS any imaginable input could be used for the counter. With a promoter activated by a heat shock protein one could for example count how many times a meal has been warmed up. Or a protein which is typical for a certain state in the cell division cycle could be used to count the number of cell divisions. With a periodical input a clock is also achievable.
Furthermore the different parts of the counter could also be reused: the 4SD (or just a part of it) for example as a simple NOR-gate or J-K-flip-flop.
Besides all these practical applications any engineer would approve that a counter is a crucial part for a finite state machine as a computer for example. Therefore this counter could be a part of the heart of a future biological computer. Such a computer can’t work as fast as common processors made of silicon. But as in a single drop millions of bacteria can exist, this drop could have the power of a huge computer network.
As already mentioned in the introduction the field of Synthetic Biology is still very young but shows a lot of potential. At this stage every new development can have huge effects on the general focusing of the field. During our project we came to the point where we had to decide whether we want to go for a cool and fancy behaviour or for a part that can be reused in the future for many other things (see the previous ideas). The result described in detail in the above sections show obviously that we went for the ladder. People who hear about Synthetic Biology for the first time by reading about our project might think: “Why should a bacterium be able to count to two?” A good question. In order to give an answer and also a feeling about the mightiness of our approach to the reader, we want to list a few of our ideas in the following:
One of the first things that comes to one’s mind is to add further 4SDs in series. This way you could not only count to 2 but to 2^n, where n is the number of 4SDs. You only have to change the zincfingerproteins of each 4SD in order to avoid interactions (for 3 fingers there are already about 250’000 possible combinations). With 64 of these 4SDs we would already have the instruction length of the latest computers.
But our counter can’t only be seen in the common sense. You can also use it to provide a certain sequence of actions. By adding to carefully selected states an extra DNA strand coding a protein one could control a complicated sequence by only giving a input signal at the event processing device. The network of 4SDs would do the rest. The network could also have parallel parts as the output of one state could be used as input of two or more other 4SDs.
As in the Registry of Standard Biological Parts the inputs and outputs of the parts are defined by PoPS any imaginable input could be used for the counter. With a promoter activated by a heat shock protein one could for example count how many times a meal has been warmed up. Or a protein which is typical for a certain state in the cell division cycle could be used to count the number of cell divisions. With a periodical input a clock is also achievable.
Furthermore the different parts of the counter could also be reused: the 4SD (or just a part of it) for example as a simple NOR-gate or J-K-flip-flop.
Besides all these practical applications any engineer would approve that a counter is a crucial part for a finite state machine as a computer for example. Therefore this counter could be a part of the heart of a future biological computer. Such a computer can’t work as fast as common processors made of silicon. But as in a single drop millions of bacteria can exist, this drop could have the power of a huge computer network.
Appendix
References
atkinson03, basu05, bates05, Beerli00 (Linker), Beerli02, Beerli98, bulter04, cho04a-sensitivity, Chou et al. 1998, Dreier01 (ANN-paper), Dreier05 (CNN-paper), goryachev05, Greisman97, Isalan01, keiler01, Klug05 (Minireview), Lai04, Mani05, miller01, Newman03 (Leucine Zippers), Park et al. 2005 (Activation of transcription), römling02, ross91, Segal03, Segal99 (GNN-paper), sudesh00, suetsugu03, sutherland01, Yang95 (Kinetics!!), you04, zogaj01
Glossary
Previous Ideas
This is the brainstorming and previous ideas section. In this section you will find other projects that had been pursued, as well as random ideas without too much consideration of feasibility, etc.