Harvard 2006
From 2006.igem.org
(→Adaptamers) |
ShawnDouglas (Talk | contribs) m (→DNA Nanobox: A Nanoscale Device for Stealth Drug Delivery) |
||
| Line 43: | Line 43: | ||
==DNA Nanobox: A Nanoscale Device for Stealth Drug Delivery== | ==DNA Nanobox: A Nanoscale Device for Stealth Drug Delivery== | ||
'''Project Overview''' | '''Project Overview''' | ||
| - | *Traditional drug delivery is | + | *Traditional drug delivery is fairly low-tech. Tablets containing a mixture of active small molecules and binders are ingested, and the small molecules circulate non-specifically throughout the body. Even when a specific cell or tissue type is targeted (e.g. cancerous cells or bacterial infections), many other tissues are exposed to the drugs, often with serious side effects. We envision a future in which medicines will be delivered in a highly specific manner to targeted tissues and cell types, and set out to make initial steps toward realizing this vision. |
*Our goal for the summer was to design and implement nanoscale molecular containers, which could be dynamically opened and closed by an external stimulus. This is the first step to making more sophisticated drug-delivery vehicles. | *Our goal for the summer was to design and implement nanoscale molecular containers, which could be dynamically opened and closed by an external stimulus. This is the first step to making more sophisticated drug-delivery vehicles. | ||
*The containers were implemented as DNA nanostructures, which afford a significant degree of positional control and chemical versatility. | *The containers were implemented as DNA nanostructures, which afford a significant degree of positional control and chemical versatility. | ||
Revision as of 01:43, 30 October 2006
| Harvard University 2006 iGEM Team | ||
Students
|
Teaching Fellows Faculty Advisors
| |
The Harvard University 2006 iGEM Team
This year Harvard's team consisted of 10 undergraduate students, with backgrounds in molecular and cellular biology, biochemistry, and computer science. With the help of five faculty advisors and three graduate-level teaching advisors, they devised and executed three separate projects.
1. Construction of novel DNA nanostructures for the purpose of stealth drug delivery,
2. Exploration of cell-surface targeting using interchangable and linkable aptamers (adaptamers) and Lpp-OmpA fusion vehicle for bacterial surface display.
3. Reconstitution of a circadian oscillator from cyanobacteria into E. coli,
DNA Nanobox: A Nanoscale Device for Stealth Drug Delivery
Project Overview
- Traditional drug delivery is fairly low-tech. Tablets containing a mixture of active small molecules and binders are ingested, and the small molecules circulate non-specifically throughout the body. Even when a specific cell or tissue type is targeted (e.g. cancerous cells or bacterial infections), many other tissues are exposed to the drugs, often with serious side effects. We envision a future in which medicines will be delivered in a highly specific manner to targeted tissues and cell types, and set out to make initial steps toward realizing this vision.
- Our goal for the summer was to design and implement nanoscale molecular containers, which could be dynamically opened and closed by an external stimulus. This is the first step to making more sophisticated drug-delivery vehicles.
- The containers were implemented as DNA nanostructures, which afford a significant degree of positional control and chemical versatility.
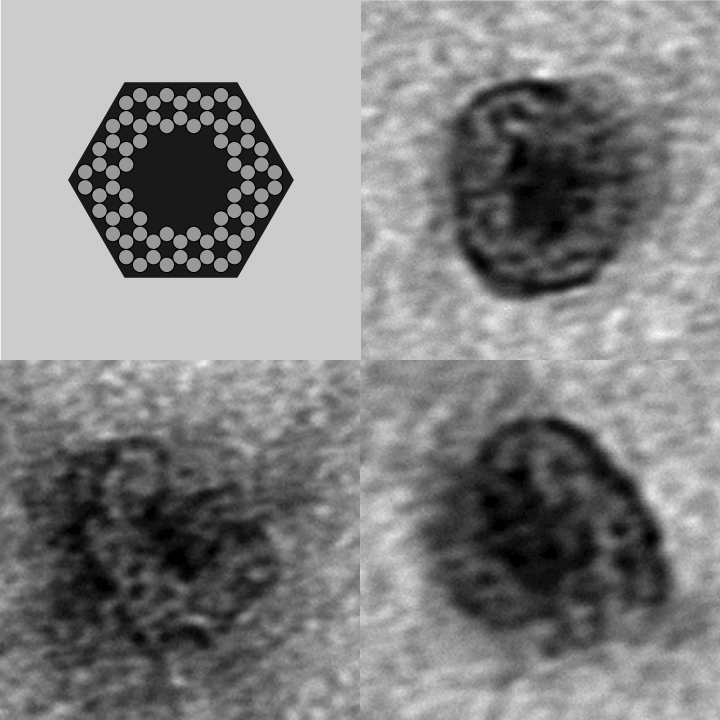
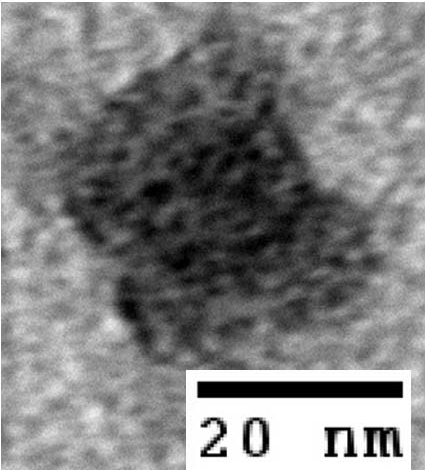
- To demonstrate that our designs successfully self-assembled, we used negative-stain electron microscopy to visualize the containers.
- As an initial proof-of-concept, we planned to demonstrate that our DNA containers could be used to "protect" biotinylated oligonucleotides from binding by streptavidin-coated magnetic beads.
- We were able to successfully show that nanoboxes with internal biotinylated oligonucleotides did not bind to streptavidin-coated magnetic beads, while nanoboxes that were externally decorated with biotinylated oligonucleotides were bound by the same beads.
Results
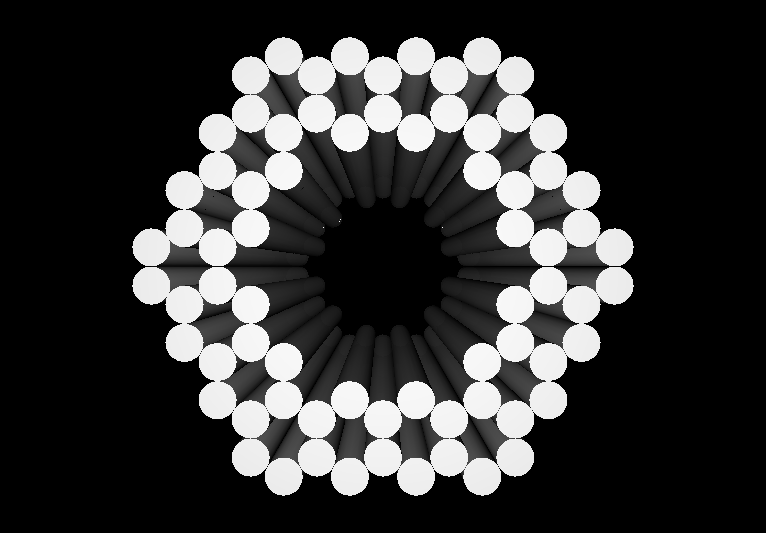
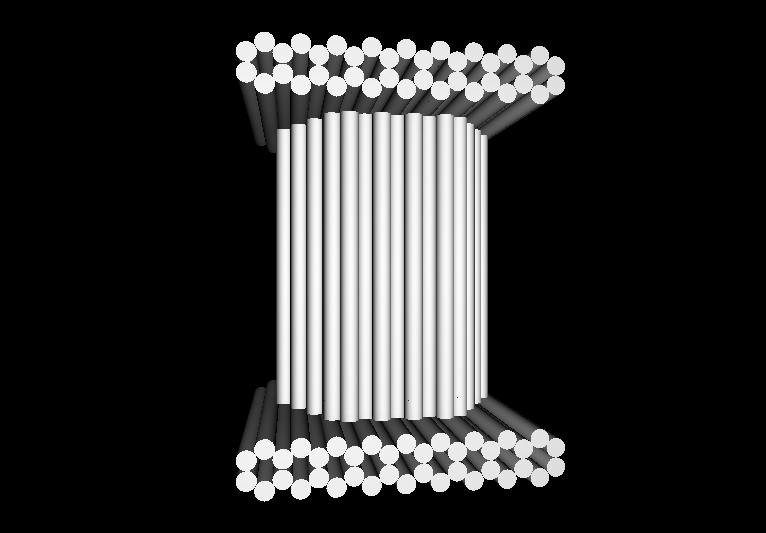
- The following images show our most successful container design, both as a 3D cartoon and in actual negative-stain EM images. The length, width, and depth of this container design is approximately 30x30x30 nanometers, so it is bigger than a typical ribosome.
- More details about this design can be found here.
- The basic design of this container is a hexagonal barrel, closed off by two flat lids.
- The lids are designed to reversibly attach to the barrel using complementary oligonucleotides using a "toehold" strategy in which the strands include unpaired overhangs that can be displaced with the addition of oligos that match for the full length of the sequence.
Future Plans
- We have demonstrated the first steps toward building a sophisticated, versatile drug delivery vehicle out of DNA.
- Short term plans include assembly of closed containers (which include lids), and further characterization of assembled structures (both imaging and functional assays). We have not determined the maximum size for a molecule that can still diffuse between DNA helices that comprise our container, but for a closed container we expect it to be around 1 nanometer in diameter.
- In the long term, we envision designing containers that incorporate cell-surface targeting mechanisms that would allow delivery of a payload to a specific cell type. Advances also need to be made if the container is going to deliver small molecules - perhaps by encapsulating the container with a liposome (or vice versa).
Electronic Notebook
Cell Surface Targeting
Adaptamers
Project Overview
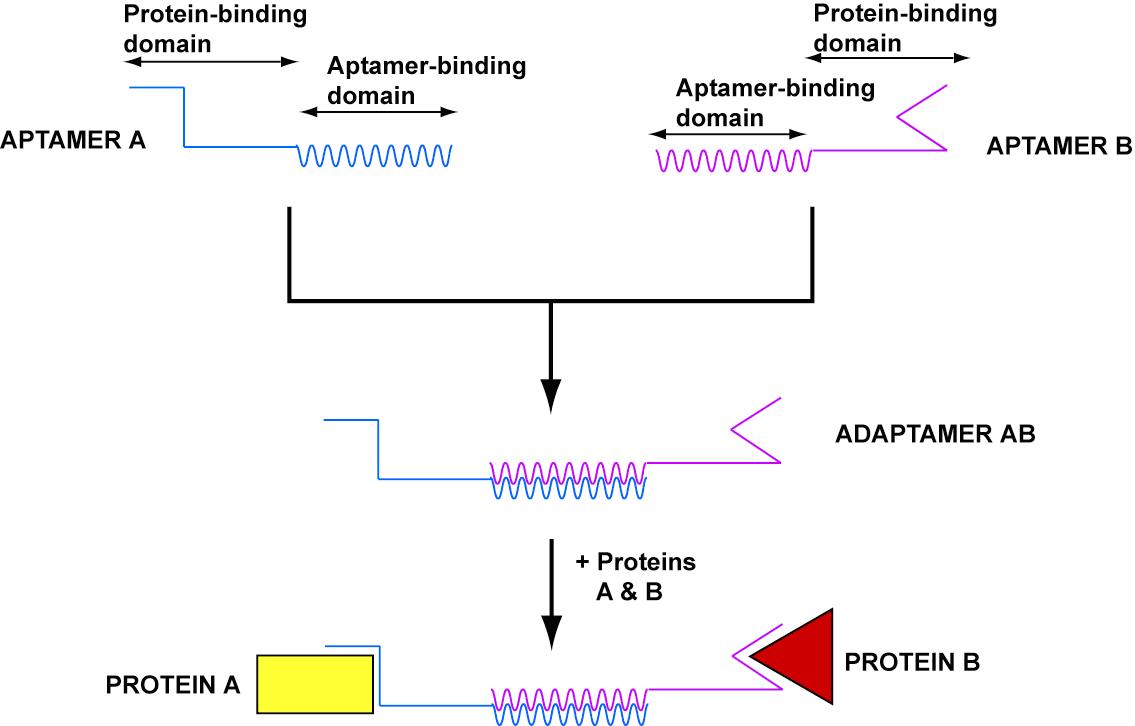
- In order to target nanostructures to cells, we developed adaptamers, universal nucleic acid adaptars which can link two substrates.
- Such an interface could also be used to link together entire cells for the study of cell-cell interactions and the linkage of two interacting proteins, in effect creating a nucleic acid enzyme.
- Adaptamers generally depend on aptamers, short sequences of nucleic acid that bind with high specificity and affinity to particular substrates.
- Tahiri-Alaoui et al. created the first aptamer in 2002, consisting of two aptamer sequences linked together by a bulky basepairing region ~100 nucleotides long.
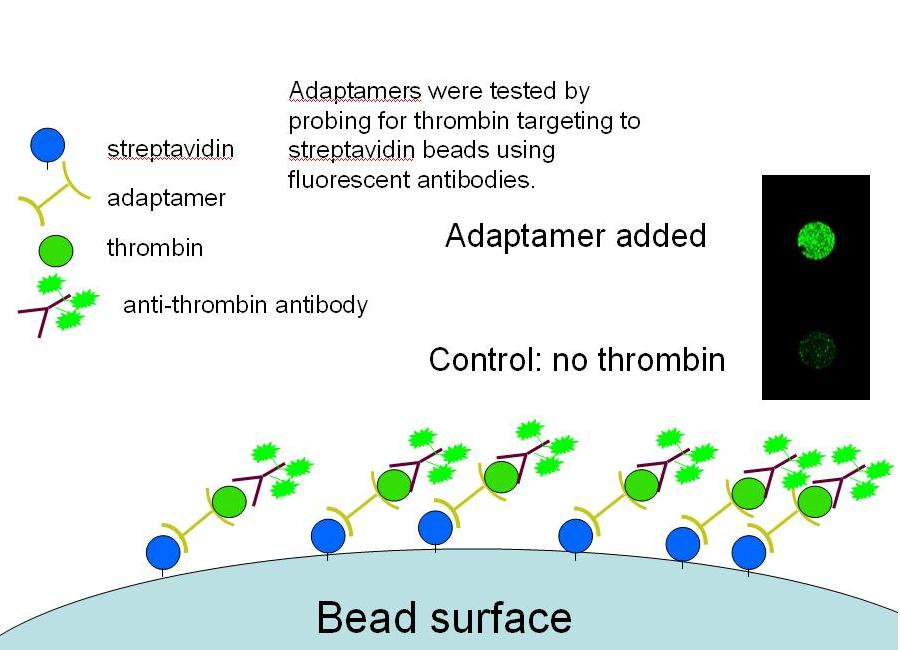
- Our goal was to create an adaptamer that could link together streptavidin and thrombin. Delivery of thrombin to a streptavidin-coated magnetic bead would show the potential for delivery of a macromolecule to a cell surface.
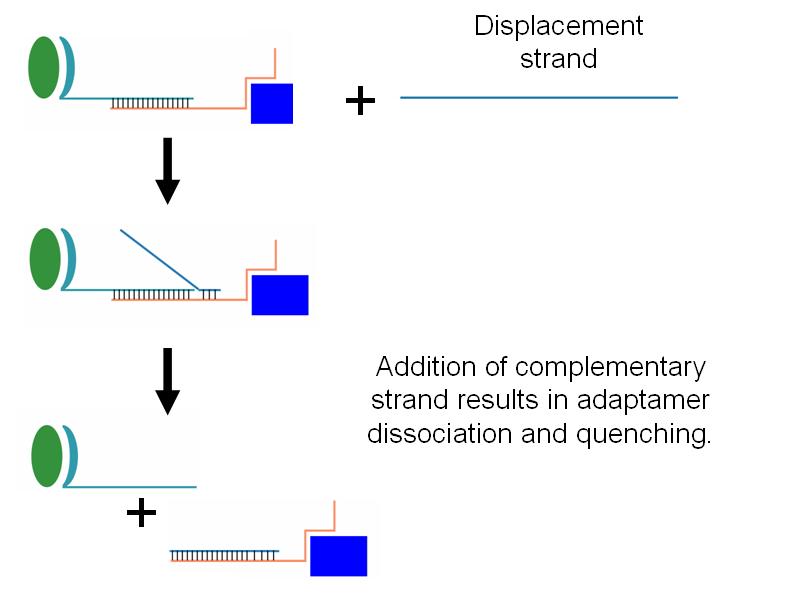
- Additionally, we wished to be able to be able to quench adaptamer function through the addition of an adapatamer-disabling oligonucleotide.
Results
- Our design consisted of a streptavidin aptamer and thrombin aptamer linked together by a short, variable-length double-stranded region of linker DNA.
- In order to prevent interaction of linker DNA with aptamers, we wrote a program that can evolve a sequence for the linking region given two aptamer sequences. This program can be used for any aptamer sequences and will be utilized for future adaptamer designs.
- We successfully showed that four separate adaptamer designs are capable of targeting thrombin to streptavidin-coated magnetic beads by probing targeted beads with thrombin antibodies.
- We successfully showed that addition of a displacement strand which breaks apart adaptamers was capable of quenching adaptamer function.
- Interestingly, high concentrations of displacement strand led to greater maintenance of thrombin targeting. We have proposed a lever-hypothesis for this observation.
Harvard2006-StrandDisplacement.jpg
Strand displacement was successful, but higher concentrations of displacement strand had opposite effect. |
Harvard2006-StrandDisplacement.jpg
Lever-hypothesis |
Future Plans
- We will demonstrate the modular design of adaptamers by using an adaptamer to target thrombin to a cell surface.
- We will attempt to prove the lever-hypothesis.
Electronic Notebook
Cell surface streptavidin
Project Overview
- The cell surface streptavidin project approaches targeting from a different direction, that is, from the cell. While the adaptamer project uses nucleic acids to target any two substrates to each other, the cell surface streptavidin project seeks to target any substrate(s) to the cell surface.
- The method of targeting would be through the expression of streptavidin protein on the cell surface. Streptavidin is a protein which binds strongly to the biotin molecule, thus there is a common practice of biotinylating (adding biotin to) nucleic acids or peptides for streptavidin affinity purification or antibody conjugation. We would be utilizing this strong streptavidin-biotin binding to target any biotinylated nucleic acid or peptide to the outside of a cell expressing streptavidin on the surface.
Results
Future Plans
Electronic Notebook
A Circadian Oscillator for E.coli
Project Overview
Results
Future Plans
Electronic Notebook
Complete Electronic Notebooks
Detailed records of our summer activities, including results, can be found on openwetware, which the team used to host its electronic notebook. Links to specific project pages can be found in the above descriptions.