Ljubljana, Slovenia 2006/Results & Conclusions
From 2006.igem.org
| Home | Background and Signalling Pathway | Project | Methods | Terms & References | Team members |
|---|
Contents |
Results
Construction of Biobricks
We prepared individual BioBricks that could be used in mammalian cells. The following BioBricks were used:
- promoter (constitutive CMV and NF-κB inducible promoter),
- terminator (which we included into plasmids to avoid repeated additional steps in construction of each functional Part
- protein coding sequences, which include:
- two inhibitory (dominant negative) proteins of the signaling cascade (dnMyD88 and dnTRAF6),
- two reporters: Renilla luciferase (in order to allow simultaneous dual luciferase assay to normalize the luminescence for the efficiency of transfection and cell number) and GFP
- PEST sequence to decrease the lifetime of the inhibitor.
Linkers between the protein coding sequences (e.g. MyD88+luciferase+PEST) were prepared by PCR ligation to avoid addition of unwanted aminoacids in the linker region between protein domains.
| Registration number | Part's Name | Part/Device | Vector |
|---|---|---|---|
| BBa_J52008 | rluc | Part | pSB1AK3 |
| BBa_J52010 | NFκB | Part | pSB1AK3 |
| BBa_J52011 | dnMyD88-likn-rLuc | Part | pSB1AK3 |
| BBa_J52012 | rluc-link-PEST191 | Part | pSB1AK3 |
| BBa_J52013 | dnMyD88-link-rluc-link-pest191 | Part | pSB1AK3 |
| BBa_J52014 | NFκB+dnMyD88-link-rLuc | Device | pSB1AK3+TER |
| BBa_J52016 | eukaryotic terminator | Part | pSB1AK3+TER |
| BBa_J52017 | eukaryotic terminator vector | pSB1AK3 | |
| BBa_J52018 | NFκB+rLuc | Device | pSB1AC3 |
| BBa_J52019 | dnTRAF6 | Part | pSB1AK3+TER |
| BBa_J52021 | dnTRAF6-link-GFP | Part | pSB1AK3+TER |
| BBa_J52022 | NFκB+dnTRAF6-link-GFP | Device | pSB1AK3 |
| BBa_J52023 | NFκB+rLuc-link-PEST191 | Device | pSB1AK3 |
| BBa_J52024 | NFκB+dnMyD88-link-rLuc-link-PEST191 | Device | pSB1AK3+TER |
| BBa_J52026 | dnMyD88-link-GFP | Part | pSB1AK3+TER |
| BBa_J52027 | NFκB+dnMyD88-link-GFP | Device | pSB1AK3 |
| BBa_J52028 | GFP-PEST191 | Part | pSB1AK3 |
| BBa_J52029 | NFκB+GFP-PEST191 | Device | pSB1AK3 |
| BBa_J52034 | CMV | Part | pSB1AK3+TER |
| BBa_J52035 | dnMyD88 | Part | pSB1AK3+TER |
| BBa_J52036 | NFκB+dnMyD88 | Device | pSB1AK3+TER |
| BBa_J52038 | CMV-rLuc | Device | pSB1AK3+TER |
| BBa_J52039 | CMV+rLuc-link-PEST191 | Device | pSB1A2 |
| BBa_J52040 | CMV+GFP-PEST191 | Device | pSB1AK3 |
| BBa_J52642 | GFP | Part | pSB1AK3+TER |
| BBa_J52648 | CMV+GFP | Device | pSB1AK3+TER |
Inducible transcription by stimulation of Toll-like receptor
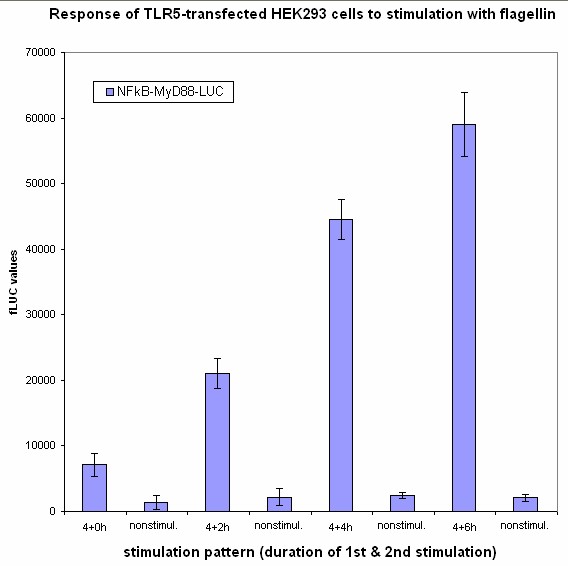
Stimulation of HEK293 cells transfected by TLR with their agonist (flagelin in case of TLR5) stimulates the translocation of NF-κB into the nucleus and activation of transcription of NF-κB-responsive genes. We have used the firefly luciferase under NF-κB dependent promoter to monitor the gene activation and tested the functionality of our part containing NF-κB dependent promotor in a construct with dnMyD88-rluc. We have detected that 4 hours (?) after cell activation we can detect the R-luciferase activity.
Inhibition of cell signaling by a feedback device
An adapter protein MyD88, consisting of a TIR and death domain, is at the crossroads of Toll-like receptors. Therefore activation of each of the surface expressed TLR recruits MyD88 to the cell membrane. We selected to use the receptor TLR5 and flagellin instead of the often used TLR4 and its agonist LPS, because the TLR4 system is extremely sensitive to the contamination with bacterial LPS, which often copurifies with the plasmid. In this way we have avoided the contamination issue, but our system is designed to work on any signalling receptor that has MyD88 in its signalling pathway (i.e. TLR1,2,4,5,6,7,8,9,11,IL-1). Cells were cotransfected with TLR5, reporter plasmid (NFκB-Fluc) and our feedback device (NFκB-dnMyD88).
We expect that after stimulation with flagellin cytoplasmic NFκB is released from its inhibitor. It then migrates into the nucleus and induces the transcription of our construct. Accumulation of the dominant negative protein should compete with wild-type MyD88 and attenuate the downstream signalling pathway. We can detect the NFκB translocation, transcriptional activation and phosphorylation by our detection systems - luminescence, free NFκB, phosphorylation detected by flow cytometry and confocal microscopy. In case that our construct is followed by PEST (degradation) sequence, it is expected that product should degrade rapidly and shorten the inhibition period. If the stimulus is still present, cells will again respond to it and the cycle with inhibition could repeat as well.
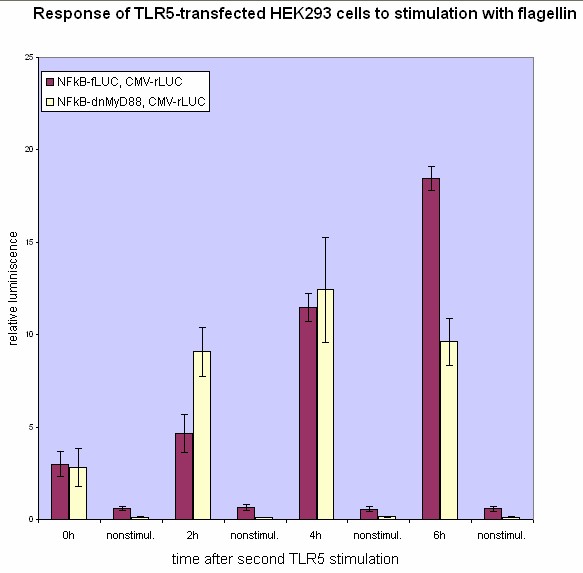
We have designed the experiment by two consecutive pulses of TLR stimulation. Normal cells should respond to both, while the cells with our device should respond equally to the first pulse -- this should stimulate the transcription of the inhibitor, therefore the response to the second stimulus should be decreased. However if the stimuli are separated by a longer span of time, the system should reset to the normal state, as the inhibitor has already degraded.
Our results show that the system with a feedback dnMyD88 indeed responds weaker to the second stimulus. The minimal delay between two stimuli should correspond to the time required for the synthesis of the inhibitor, which is adequate since it does not completely disrupt the cellular response but decreases it after extended stimulation.
Decrease of the protein lifetime by the PEST sequence
Rapid degradation signature motif - "PEST" was considered to tune the lifetime of the inhibitor and to reset the cells to the normal responsiveness. Fusion of the luciferase and PEST sequence under the control of CMV (Part ?) was used to test the effectiveness of the PEST sequence. We have used the cycloheximide toblock the protein synthesis and analyse the lifetime of the fusion protein with PEST sequence. Constructs of the inhibitory domain and PEST sequence were not effective because of the infetference of the C-terminal extension of the inhibitory domain, as described above for the fusion constructs with reporter luciferase or GFP.
Conclusions
- the same principles of BioBricks can be used in the mammalian cell system as in bacteria and eucaryotes
- we have succesfully implemented a feedback loop that decreases the cellular activation with some delay
- response of this feedback loop is transient and the cell responsiveness is restored after the synthesized inhibitor has degraded
- dnMyD88 inhibitor was not effective as a C-terminal fusion with luciferase or GFP, which may be due to the steric hindrance; N-terminal fusion with reporter might be functional
- our constructed device mimicks the natural mechanism of tolerance only that it is activated faster, which may be a benefit for organism,
- simplified model of the TLR signaling qualitatively captures most of the features of the natural system
Suggestions
- for mammalian cell systems an additional set of vectors may be constructed based on the backbone, which includes the selection marker for stable transfection or retroviral integration
| Home | Background and Signalling Pathway | Project | Methods | Terms & References | Team members |
|---|