IAP2004:Polkadorks
From 2006.igem.org
(→System Diagram) |
(→Device Diagram) |
||
| Line 39: | Line 39: | ||
[[Image:Intro21-SystemDiagram.jpg]] | [[Image:Intro21-SystemDiagram.jpg]] | ||
| - | === Device Diagram === | + | === Parts-Level Device & System Diagram === |
[[Image:Intro22-DeviceDiagram.jpg]] | [[Image:Intro22-DeviceDiagram.jpg]] | ||
| - | |||
=== Layout Diagram === | === Layout Diagram === | ||
Latest revision as of 22:22, 20 July 2006
Polkadorks and the E. Coli-brator
est. January 5, 2004
Dorks: Ziyan Chu, Roshan Kumar, Stephen Lee, Joe Levine
Background
Last year's groups were asked to create systems that blink; this year our job was to create systems that form a spatial design. We, the Polkadorks, chose polkadots for our design and have spent the month of January 2004 working on this system.
System Overview
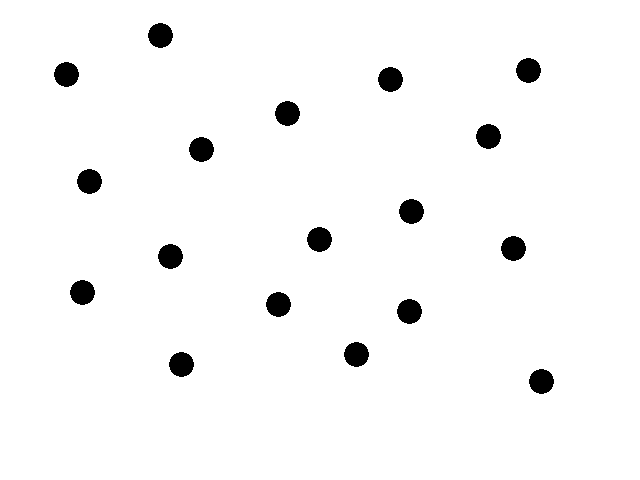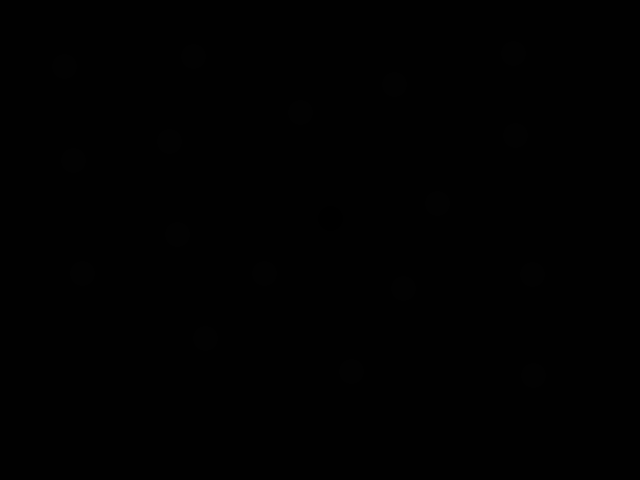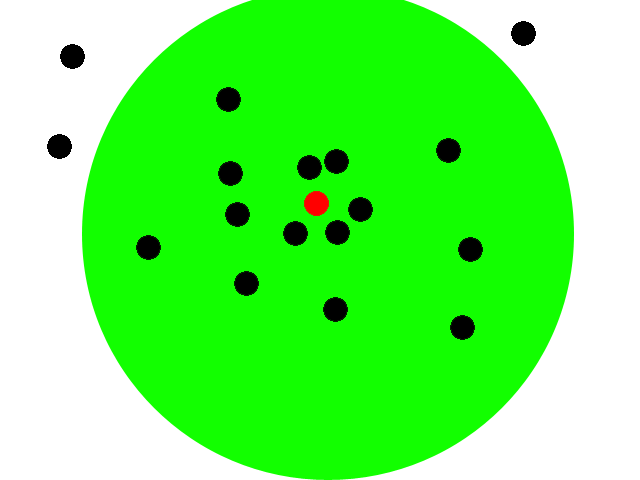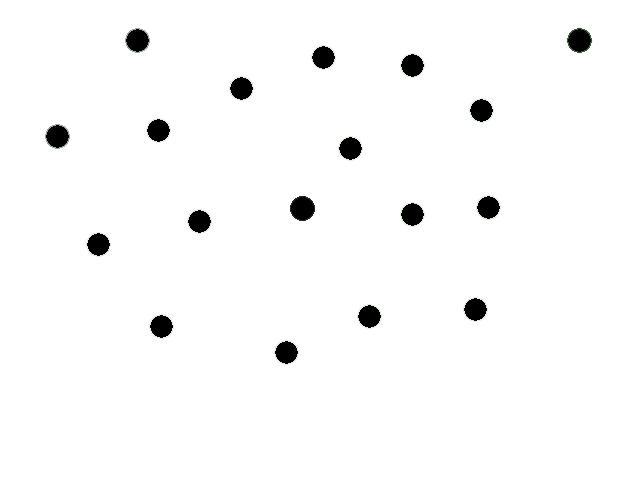
This animation illustrates our basic system. We start with a collection of engineered e-coli moving randomly in plated media. The bacteria are represented by black dots in the animation. Under control of a stochastic element, a few begin excreting an attractant. These bacteria we will call the sender cells. In the movie we have one sender cell represented by the red dot; the green circle represents the attractant diffusing away from the sender cell.
Through chemotaxis, a process by which a cell along a chemical gradient swims toward or away from the stimulus (an attractant in this case), nearby bacteria start swimming towards the sender cells. These bacteria we will call the receiver cells. This way, groups begin forming around the original sender cells on the plate.
All bacteria have been engineered with a quorum sensing mechanism which effectively senses local cell density. In the groups that have formed on our plate, the cell density eventually reaches a certain threshold. The quorum sensing mechanism of the cells then stops secretion of any attractant. The existing attractant then diffuses away. Since there is no more attractant being secreted, the cells will diffuse away and eventually be spread out across the plate once again. Then by the stochastic element a few cells will begin excreting the attractant and the whole process is repeated.
Basically polkadots will form, diffuse, and form again in random areas on the plate. Our system should thus form time-varying patterns based on local random time-varying symmetry breaking.
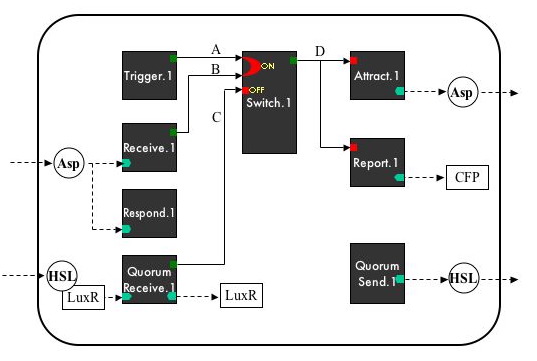
Device-Level System Diagram
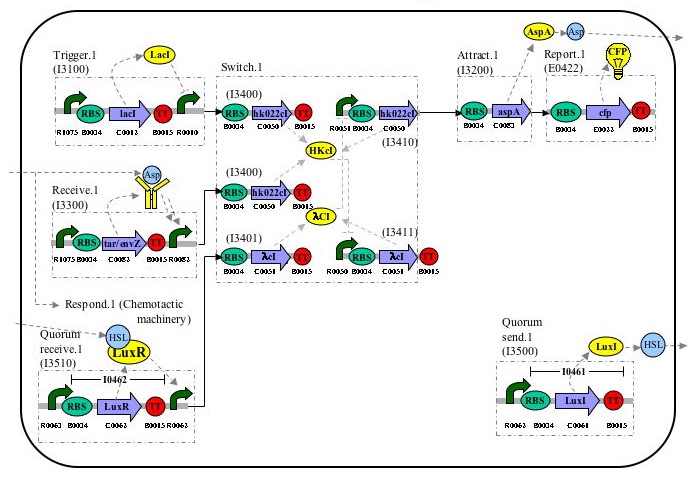
Parts-Level Device & System Diagram
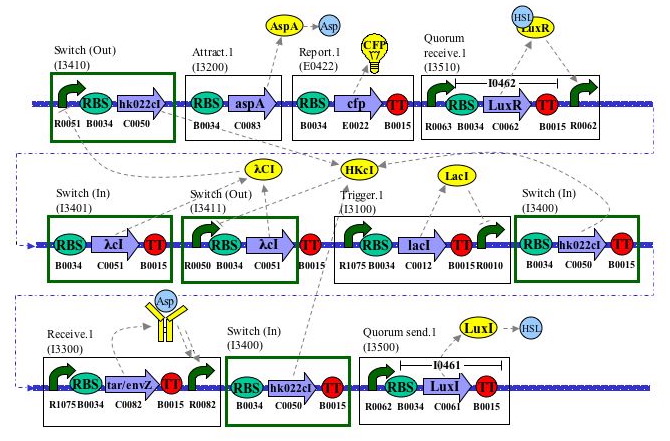
Layout Diagram
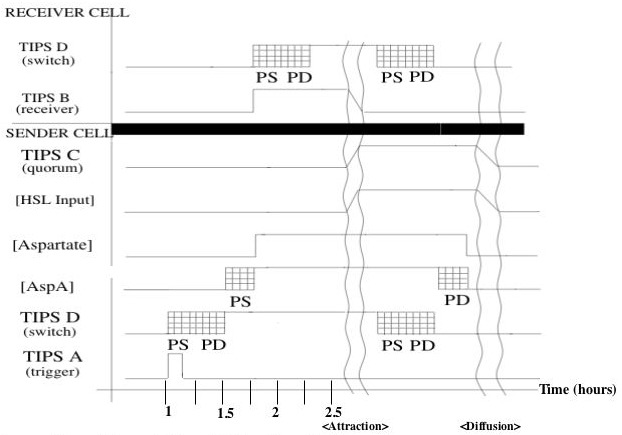
Timing Diagram
Shown is a putative timing diagram of our system for a typical attractant cycle. At some time the trigger activates randomly, sending a TIPS signal to the switch. The switch output rises with a delay set by protein synthesis and degredation (to be explained in the switch device section). This causes the AspA level to rise with another protein synthesis delay. The corresponding increase in Aspartate will activate the switches of other cells (receiver cell in the diagram). After an undefined attraction period, the cells will have formed a dense enough cluster to activate quorum sensing. The switch will turn off with a similar protein synthesis/degredation delay, and aspartate will begin to diffuse away. The cell cluster will diffuse away, at which point the system will be reset to its initial conditions.
Population Simulations
Quicktime movies not yet loaded...
Characterization
We have specified various protocols to validate our system part by part. We will validate each individual device in our block diagram separately and then subsequently test compound parts. Our plans for device testing are listed below.
Testing Single Devices
*Trigger *Switch *Aspartate Sender *Aspartate Receiver *Quorum Sender *Quorum Receiver
Testing Compound Devices
*Aspartate Sender & Switch *Aspartate Receiver & Switch *Quorum Receiver & Switch
Testing Collective Behaviors
*Attractant-Chemotaxis Interaction *Characterizing Quorum Response to Clustering
Parts List
| Systems Device | Device Name | Part Description | BioBricks Number |
| Trigger.1 | Lac stochastic trigger device | BBa_I3100 | |
| Constitutive Promoter | BBa_R1075 | ||
| RBS | BBa_B0034 | ||
| LacI CDS | BBa_C0012 | ||
| TT | BBa_B0015 | ||
| LacI Promoter | BBa_R0010 | ||
| Receive.1 | Tar-EnvZ attractant receiver device | BBa_I3300 | |
| Constitutive Promoter | BBa_R1075 | ||
| RBS | BBa_B0034 | ||
| Tar-EnvZ CDS | BBa_C0082 | ||
| TT | BBa_B0015 | ||
| OmpR Promoter | BBa_R0082 | ||
| Quorum Receive.1 | |||
| Quorum receiver device | BBa_I3510 | ||
| Lux pL Promoter | BBa_R0063 | ||
| LuxR Device | BBa_I0462 | ||
| Lux pR Promoter | BBa_R0062 | ||
| Switch.1 | |||
| Switch In (On) | Hk022cI switch input device | BBa_I3400 | |
| RBS | BBa_B0034 | ||
| Hk022cI CDS | BBa_C0050 | ||
| TT | BBa_B0015 | ||
| Switch In (Off) | Lambda cI switch input device | BBa_I3401 | |
| RBS | BBa_B0034 | ||
| Lambda cI CDS | BBa_C0051 | ||
| TT | BBa_B0015 | ||
| Switch Out (On) | Hk022cI switch output device | BBa_I3410 | |
| Promoter | BBa_R0051 | ||
| RBS | BBa_B0034 | ||
| hk022cI CDS | BBa_C0050 | ||
| Switch Out (Off) | Lambda cI switch output device | BBa_I3411 | |
| Promoter | BBa_R0050 | ||
| RBS | BBa_B0034 | ||
| Lambda cI CDS | BBa_C0051 | ||
| Attract.1 | AspA attractant sender device | BBa_I3200 | |
| RBS | BBa_B0034 | ||
| AspA CDS | BBa_C0083 | ||
| Report.1 | CFP Reporter Device | BBa_E0422 | |
| RBS | BBa_B0034 | ||
| CFP CDS | BBa_E0022 | ||
| TT | BBa_B0015 | ||
| Quorum Send.1 | Quorum sender device | BBa_I3500 | |
| Lux pR Promoter | BBa_R0062 | ||
| LuxI Device | BBa_I0461 |
References
- Betterton MD and Brenner MP. Collapsing Bacterial Cylinders. Physical Review E v64 pp061904-061918.
- Brenner MP, Levitov LS, Budrene EO. Physical Mechanisms for Chemotactic Pattern Formation by Bacteria. Biophysical Journal. v74 1677-1693, April 1998.
- NBudrene EO, Berg HC. Dynamics of formation of symmetrical patterns by chemotactic bacteria. Nature 376, 49-53 (1995).
- Elowitz M and Liebler S. A synthetic oscillatory network of transcriptional regulators. Nature 403:335-338 (2000).
- Forst SA, Delgado J, Inouye M. DNA-binding properties of the transcription activator (OmpR) for the upstream sequences of ompF in Escherichia coli are altered by envZ mutations and medium osmolarity. Journal of Bacteriology. 171 (6), 2949-2955 (1989)
- Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature 403:339:342 (2000).
- Maeda S and Mizuno T. Evidence for multiple OmpR binding sites in the upstream activation sequence of the OmpC promoter in Excherichia coli: a single OmpR-binding site is capable of activating the promoter. Journal of Bacteriology. 172 (1), 501-503 (1990).
- Mittal N, Budrene EO, Brenner MP, van Oudenaarden A. Motility of Escherichia coli cells in clusters formed by chemotactic aggregation. Proceedings of the National Academy of Sciences, v100 n23, Nov 11 2003 pp 13259-13263.
- Utsumi R, Brissette RE, Rampersaud A, Forst SA, Oosawa K, Inouye M. Activation of bacterial porin gene expression by a chimeric signal transducer in response to aspartate. Science v245 pp1246-1249 (1989)
- Weiss, R. Cellular Computation and Communications using Engineered Genetic Regulatory Networks. MIT EECS PhD Thesis. August, 2001.