Synchronization of Cell Cycles
From 2006.igem.org
(→Synchronization of Cell Cycles) |
(→Constructs) |
||
| (6 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
===Aim=== | ===Aim=== | ||
| - | + | Our aim is to develop a synthetic gene network to synchronize ''E. coli'' cell cycles. | |
| - | + | ||
===Approach=== | ===Approach=== | ||
| + | Cell cycle is a robust biological oscillator. Theoretical work predicts that weak coupling of cellular oscillators should result in their synchronization [Garcia-Ojalvo et al., 2005]. Our core oscilltor is a cell cycle phase dependant promoter- Pcya. | ||
| + | We plan to couple these oscillators using LuxI-LuxR based quorum sensing signaling machinery.The core oscilltor will regulate expression luxI(an enzyme that produces an autoinducer acyl-homoserine-lactone).Oscilltory expression of luxI would result in oscilltory production of the autoinducer(AI). This in turn would cause oscillatory expression from a luxR-AI dependant promoter. | ||
| + | |||
| + | The cell cycle phase will be modulated using DnaA sequestration. DnaA is a cell cycle regulator in ''E.coli''. Cell cycle progression is affected by decrease in free and active DnaA molecules is a cell. DnaA temperature sensitive mutants are used for cell cycle synchronization. We have used DnaA binding sites on plasmid to 'sequester' free DnaA molecules. We expect transcription from upstream promoter would change concentration of free DnaA inside a cell. We have put luxR dependant promoter upstream to DnaA binding sites. Thus, concentration of free DnaA concentration is dependant on AI concentation. This would lead to regulation of local cell cycle phase based on global phase. | ||
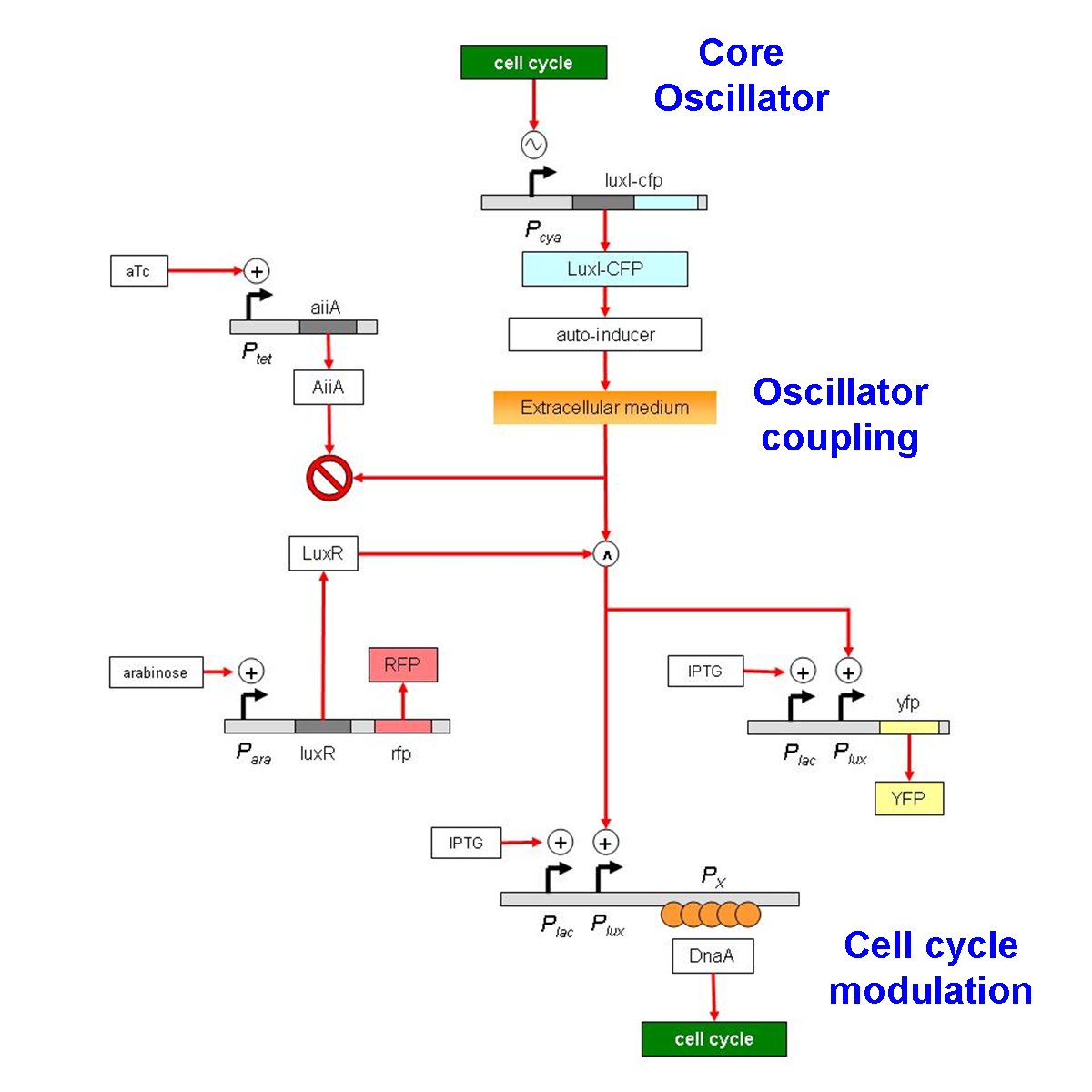
===Constructs=== | ===Constructs=== | ||
| - | + | [[Image:Sync circuit.png|thumb|left|Synchronization circuit]] | |
| + | |||
| + | Core oscillator: The E.coli cell cycle | ||
| + | |||
| + | Pcya regulate expression of LuxI-cfp(LVA) fusion protein. LuxI is fused to cfp(LVA) in order to monitor its expression and presence of LVA tag on the fusion protein would reduce the half life. This would increse peak to trough ratio. | ||
| + | |||
| + | |||
| + | Oscillator coupling: Vibrio quorum sensing machinery | ||
| + | |||
| + | Oscillators are coupled by feeding the oscillatory signal through the quorum sensing circuit. AiiA an intracellular protein that drgrades AI is expresed from an inducible promoter in order to increase peak to trough ratio for AI. | ||
| + | |||
| + | Cell cycle modulation: DnaA sequestration | ||
| + | DnaA is cell cycle regulator in ''E. coli''. We have used DnaA binding sites downstream to pLux and pLac promoters to regulate free DnaA concentration. | ||
===Testing oscillations=== | ===Testing oscillations=== | ||
Latest revision as of 18:57, 30 October 2006
Contents |
Group II
Aim
Our aim is to develop a synthetic gene network to synchronize E. coli cell cycles.
Approach
Cell cycle is a robust biological oscillator. Theoretical work predicts that weak coupling of cellular oscillators should result in their synchronization [Garcia-Ojalvo et al., 2005]. Our core oscilltor is a cell cycle phase dependant promoter- Pcya.
We plan to couple these oscillators using LuxI-LuxR based quorum sensing signaling machinery.The core oscilltor will regulate expression luxI(an enzyme that produces an autoinducer acyl-homoserine-lactone).Oscilltory expression of luxI would result in oscilltory production of the autoinducer(AI). This in turn would cause oscillatory expression from a luxR-AI dependant promoter.
The cell cycle phase will be modulated using DnaA sequestration. DnaA is a cell cycle regulator in E.coli. Cell cycle progression is affected by decrease in free and active DnaA molecules is a cell. DnaA temperature sensitive mutants are used for cell cycle synchronization. We have used DnaA binding sites on plasmid to 'sequester' free DnaA molecules. We expect transcription from upstream promoter would change concentration of free DnaA inside a cell. We have put luxR dependant promoter upstream to DnaA binding sites. Thus, concentration of free DnaA concentration is dependant on AI concentation. This would lead to regulation of local cell cycle phase based on global phase.
Constructs
Core oscillator: The E.coli cell cycle
Pcya regulate expression of LuxI-cfp(LVA) fusion protein. LuxI is fused to cfp(LVA) in order to monitor its expression and presence of LVA tag on the fusion protein would reduce the half life. This would increse peak to trough ratio.
Oscillator coupling: Vibrio quorum sensing machinery
Oscillators are coupled by feeding the oscillatory signal through the quorum sensing circuit. AiiA an intracellular protein that drgrades AI is expresed from an inducible promoter in order to increase peak to trough ratio for AI.
Cell cycle modulation: DnaA sequestration
DnaA is cell cycle regulator in E. coli. We have used DnaA binding sites downstream to pLux and pLac promoters to regulate free DnaA concentration.
Testing oscillations
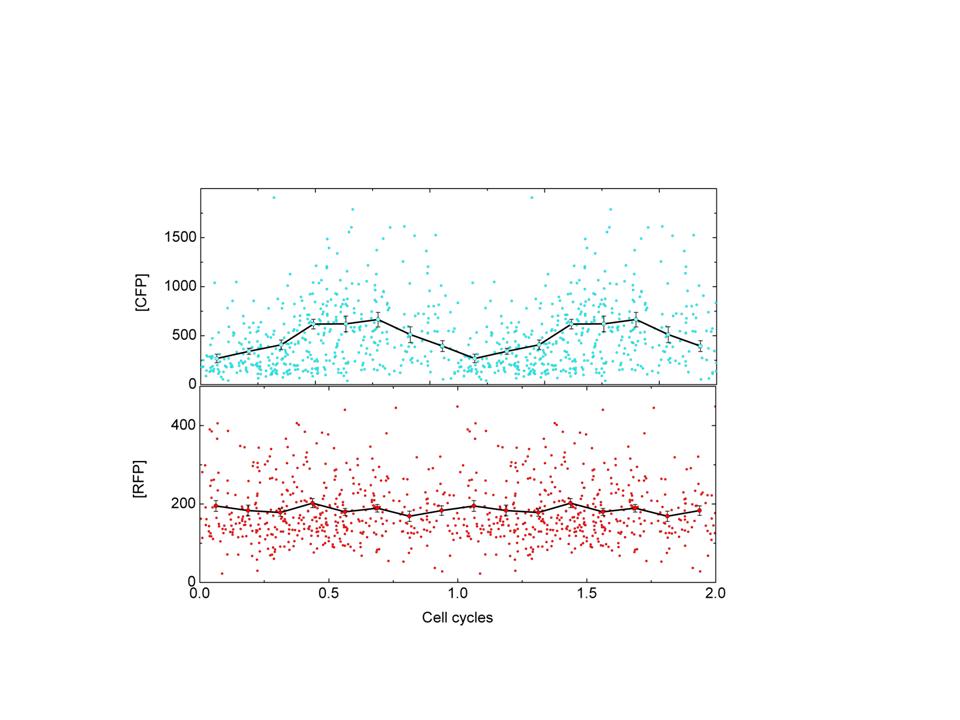
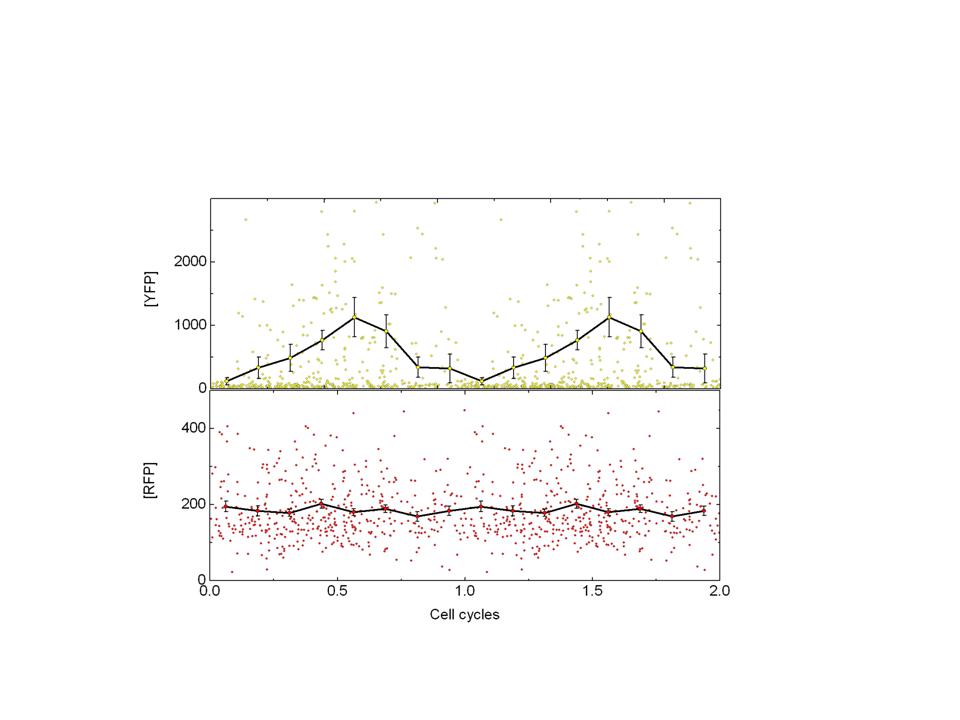
LuxI tagged with cfp(+LVA) is expressed from this promoter.LuxI oscillations will result in AI(autoinducer) oscillations and this would result in oscillations of AI dependant promoter. YFP is expressed from such a promoter. CFP expression is readout for the core oscillator-Pcya and YFP is readout of the expression of the quorum sensing promoter-Plux.
Y axis is the mean cfp fluorescence of a cell. X-axis is a correlate of cell cycle phase.Here, we have used cell length as a crude correlate for cell cycle phase.The same data is platted twice for visual representation of oscillation. RFP expression is control.
Y axis is the mean yfp fluorescence of a cell. X-axis is major axis length of a cell which is a correlate of cell cycle phase.
--Sugat 06:49, 8 July 2006 (EDT)