Memory effects of UV exposure
From 2006.igem.org
(→Constructs) |
(→Conclusions) |
||
| (7 intermediate revisions not shown) | |||
| Line 41: | Line 41: | ||
[[Image:lac circuit in muk21.jpg |thumb|center|250 px| The lac circuit in Muk21 [Ozbudak EM et. al., 2004] ]] | [[Image:lac circuit in muk21.jpg |thumb|center|250 px| The lac circuit in Muk21 [Ozbudak EM et. al., 2004] ]] | ||
| + | The regulatory network involved in uptake and utilization of lactose exhibits bistability over a range environmental conditions [Ozbudak et al., 2004]. This has been demonstrated in an ''E.coli'' strain Muk21 that has chromosomal integration of GFP expressed under lac promoter. | ||
| + | |||
| + | A bistable network is hysteretic. Environmental history determines state of the network at intermediate inducer concentration. | ||
| + | [[Image:bistability 1.jpg |thumb|center| Hysteretic behavior of the lac circuit [Ozbudak EM et. al., 2004]]] | ||
=== Constructs === | === Constructs === | ||
| Line 46: | Line 50: | ||
Here comes the exciting stuff, the synthetic biology part! | Here comes the exciting stuff, the synthetic biology part! | ||
| - | [[Image:parts group3.jpg |thumb|center|300 px|Parts|right]] We have placed the lacY gene under the SOS (UV responsive) promoter. Since the | + | [[Image:parts group3.jpg |thumb|center|300 px|Parts|right]] We have placed the lacY gene under the SOS (UV responsive) promoter. Since the effect of build up of Lac Y upon our memory switch would take time, we needed an immediate reporter of promoter activity . So the mRFP gene was also placed under the SOS promoter to make the final part - mRFP and LacY gene under the SOS promoter. |
| Line 57: | Line 61: | ||
'''Logic of this circuit.''' | '''Logic of this circuit.''' | ||
| - | Upon UV exposure the SOS promoter is active and drives the production of Lac Y.(measurement of RFP fluorescence levels gives the immediate measure of promoter activity) The | + | |
| + | Upon UV exposure the SOS promoter is active and drives the production of Lac Y.(measurement of RFP fluorescence levels gives the immediate measure of promoter activity) The change in the levels of Lac Y will drive the production of chromosomal GFP and thus cells that were UV damaged (SOS promoter active) would fluoresce Green and others which didn't would be non green. | ||
===Testing Bisability in the UV-switch strain=== | ===Testing Bisability in the UV-switch strain=== | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| Line 74: | Line 74: | ||
[[Image:UV-bistab2.jpg |thumb|center| Bistability experiment in UV-switch strain]] | [[Image:UV-bistab2.jpg |thumb|center| Bistability experiment in UV-switch strain]] | ||
| - | This is a scatter plot of cells. Cells with uninduced ( | + | This is a scatter plot of cells. Cells with uninduced (left panel) or induced (right panel) history are grown in different TMG concentrations. |
| + | |||
| + | === Conclusions === | ||
| + | |||
| + | Synthetic components can interact with the host in unanticipated ways. Extrinsic LacI binding sites destroy bistability of the lactose uptake module. | ||
Latest revision as of 07:05, 31 October 2006
Contents |
Group III
Aim
We are interested in converting a transient UV exposure into a persistent response. We plan to achieve this by coupling UV response system to a bistable network. Such system can be used to monitor cells that have undergone UV damage.
Approach
So what is this UV damage response and why long term monitoring?
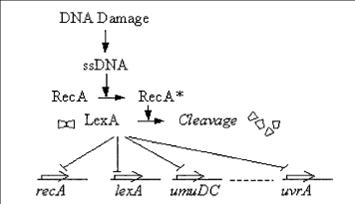
The SOS response
The SOS genetic network consists of various genes that perform diverse functions in response to DNA Damage NER (Nucleotide Excision Repair) Translesion DNA replication Homologous recombination Cell division arrest
RecA acts as a sensor of DNA Damage RecA gets activated and mediates LexA auto-cleavage
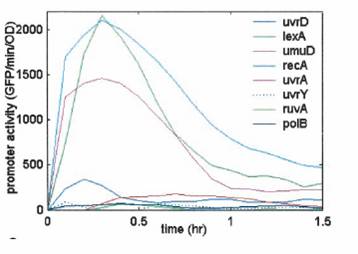
Transient response induced in response to DNA damage. The promoter activity increases rapidly in the first few minutes and decrease within an hour.
Thus, long time monitoring of DNA Damage induced by UV Irradiation requires that this transient signal generated is stabilized.
So, we tried to couple this transient signal to a bistable network to enable long time monitoring of DNA Damage.
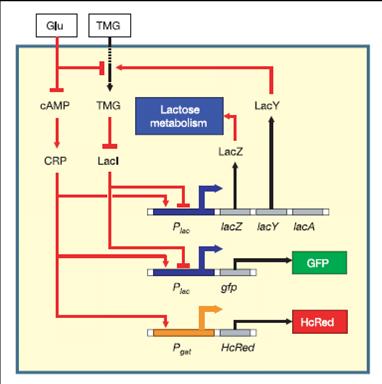
We have used the lac regulatory network as the bistable network.
The regulatory network involved in uptake and utilization of lactose exhibits bistability over a range environmental conditions [Ozbudak et al., 2004]. This has been demonstrated in an E.coli strain Muk21 that has chromosomal integration of GFP expressed under lac promoter.
A bistable network is hysteretic. Environmental history determines state of the network at intermediate inducer concentration.
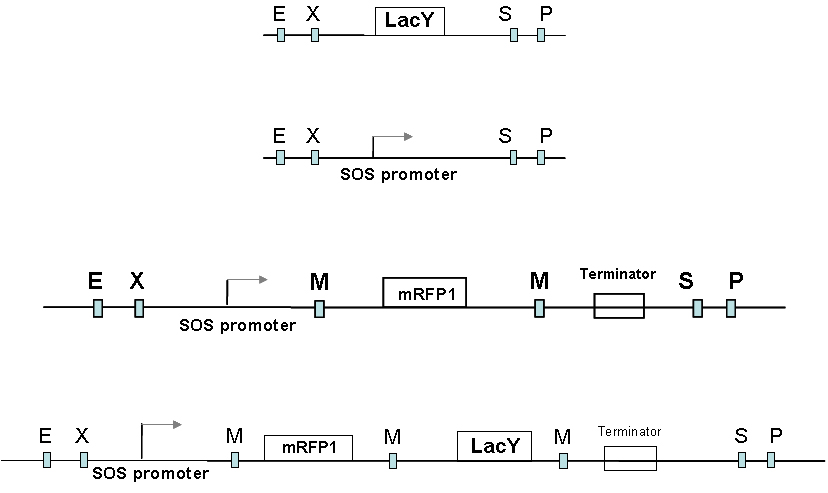
Constructs
Here comes the exciting stuff, the synthetic biology part!
We have placed the lacY gene under the SOS (UV responsive) promoter. Since the effect of build up of Lac Y upon our memory switch would take time, we needed an immediate reporter of promoter activity . So the mRFP gene was also placed under the SOS promoter to make the final part - mRFP and LacY gene under the SOS promoter.
This is then transformed into the Muk21 strain to make the final UV-switch circuit.
Logic of this circuit.
Upon UV exposure the SOS promoter is active and drives the production of Lac Y.(measurement of RFP fluorescence levels gives the immediate measure of promoter activity) The change in the levels of Lac Y will drive the production of chromosomal GFP and thus cells that were UV damaged (SOS promoter active) would fluoresce Green and others which didn't would be non green.
Testing Bisability in the UV-switch strain
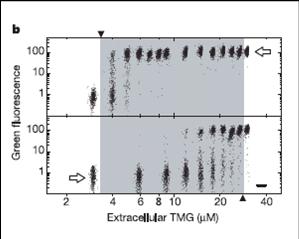
We transformed Muk21 with our UV-switch construct to test hysteresis which is a characteristic behavior of a bistable network.
This is a scatter plot of cells. Cells with uninduced (left panel) or induced (right panel) history are grown in different TMG concentrations.
Conclusions
Synthetic components can interact with the host in unanticipated ways. Extrinsic LacI binding sites destroy bistability of the lactose uptake module.