University of Texas 2006
From 2006.igem.org
| (31 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
[[Image:FSMcrop.jpg|center|300px|]] | [[Image:FSMcrop.jpg|center|300px|]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
The UT Austin team is: | The UT Austin team is: | ||
* Aaron Chevalier | * Aaron Chevalier | ||
* Eric Davidson | * Eric Davidson | ||
| - | * [http://openwetware.org/wiki/User: | + | * Bryan Kaehr |
| - | + | * [http://openwetware.org/wiki/User:LLavery Laura Lavery] | |
* Matt Levy | * Matt Levy | ||
* [http://www.mine-control.com/ Zack Booth Simpson] | * [http://www.mine-control.com/ Zack Booth Simpson] | ||
| + | * [http://openwetware.org/wiki/User:Jeff_Tabor Jeff Tabor] | ||
| + | |||
Advisors: | Advisors: | ||
* [http://ellingtonlab.org/ Andy Ellington] | * [http://ellingtonlab.org/ Andy Ellington] | ||
* [http://polaris.icmb.utexas.edu/home.html Edward Marcotte] | * [http://polaris.icmb.utexas.edu/home.html Edward Marcotte] | ||
| + | * [http://research.cm.utexas.edu/jshear Jason Shear] | ||
| - | |||
| - | <div>For the 2004 Synthetic Biology Competition, our joint UT-Austin/UCSF team designed a genetically encoded edge detection device. In our system, a community of genetically identical ''E.coli'' would function as a massively parallel computer capable of calculating the light/dark boundary of an arbitrary two-dimensional image. | + | ==Previous work: Bacterial Photography== |
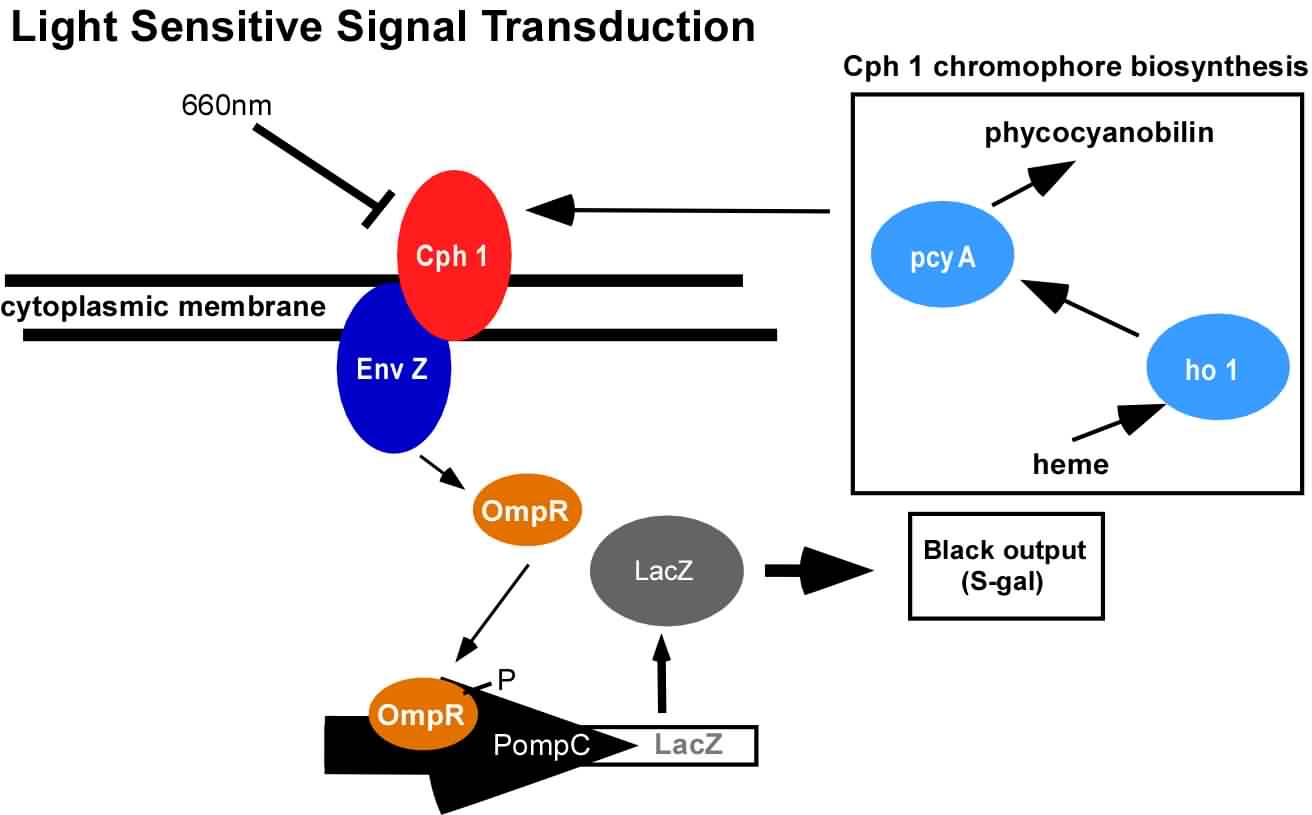
| + | [[Image:signal_transduction(S-Gal)_3.jpg|thumb|right|400px| In the dark, OmpR is phosphorylated, LacZ is expressed, and a sugar (S-Gal) in the medium is cleaved to leave a black chemical deposit. This signal transduction cascade is repressed in the presence of red light]] | ||
| + | [[Image:darwin.JPG|thumb|left|220px| Chucky D. ''Bacterial photo: Aaron Chevalier'']] | ||
| + | <div>For the 2004 Synthetic Biology Competition, our joint UT-Austin/UCSF team designed a genetically encoded edge detection device. In our system, a community of genetically identical ''E.coli'' would function as a massively parallel computer capable of calculating the light/dark boundary of an arbitrary two-dimensional image. | ||
| - | + | An obvious hurdle in the implementation of this system was genetically encoding light detection in the naturally blind organism, ''E.coli''. To accomplish this, we used a synthetic part engineered in the Voigt lab. This part, Cph8, ([http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=5302 I15010]) is an engineered fusion between a cyanobacterial light sensing phytochrome (Cph1) and an ''E.coli'' transmembrane histidine kinase, (EnvZ). 660nm light causes an isomerization in the Cph1 domain of the chimera which inactivates the histidine kinase acitity of EnvZ. When EnvZ kinase activity is inhibited, a cascade which activates transcription from the ''OmpC'' promoter [http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=3910 R0082]) is also inhibited, while transcription from the ''OmpF'' promoter ([http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=3915 R0084]) is activated. | |
| + | We then demonstrated that when this system is expressed in ''E.coli'', it is possible to transform each cell on an agar surface into a decision making pixel capable of reporting whether it is in the light or dark. The community of cells is therefore capable of genetically reproducing a light image. | ||
| - | + | This year, we have added to our previous work with Cph8 by further characterizing its response under varying conditions, including the effect of osmolarity and different cell strains. Further, it has been known that the chromophore responds in opposite ways to light around 660 versus 735nm. Previously, light around 660nm was used to repress signal transduction. We now show that light around 735nm can be used to further activate signal transduction. If both lights are used simultaneously, an increase in contrast can be achieved. We have also characterized the one-plasmid biobrick vector containing the genes for both Cph8 and the biosynthesis of the chromophore. | |
| - | + | ||
| - | |||
| + | ==Current work: Edge detector== | ||
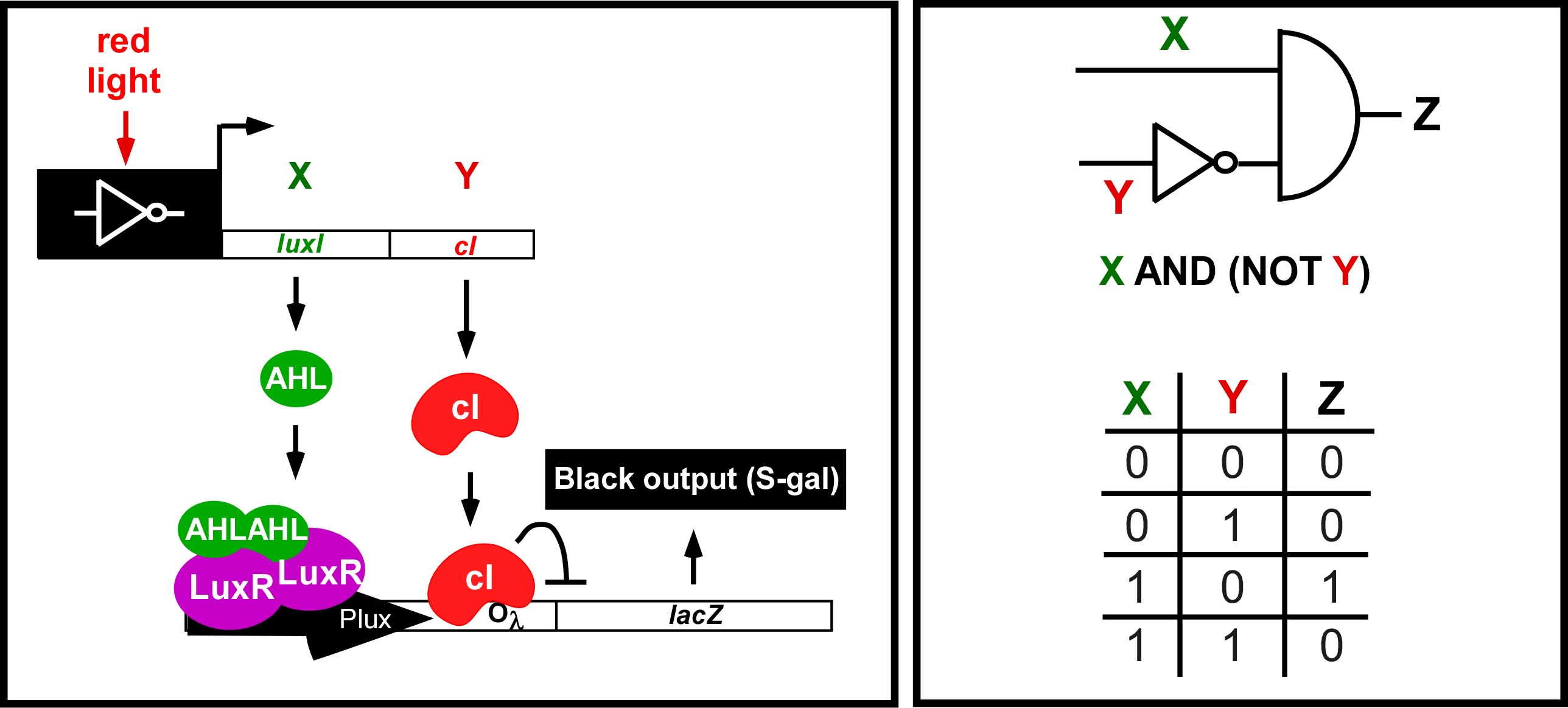
| + | <div>Bacterial Photography was the first step in engineering the edge detector. We have now "black boxed" the light-system and used it as an input for the of the edge detection circuitry ([http://partsregistry.org/Part:BBa_I15022 I15022]). The schematic and operation of the edge detector are shown in the figures below. '''<font color = "red">Please attend our iGEM2006 talk to see the results of our efforts!...</font>'''</div> | ||
| - | |||
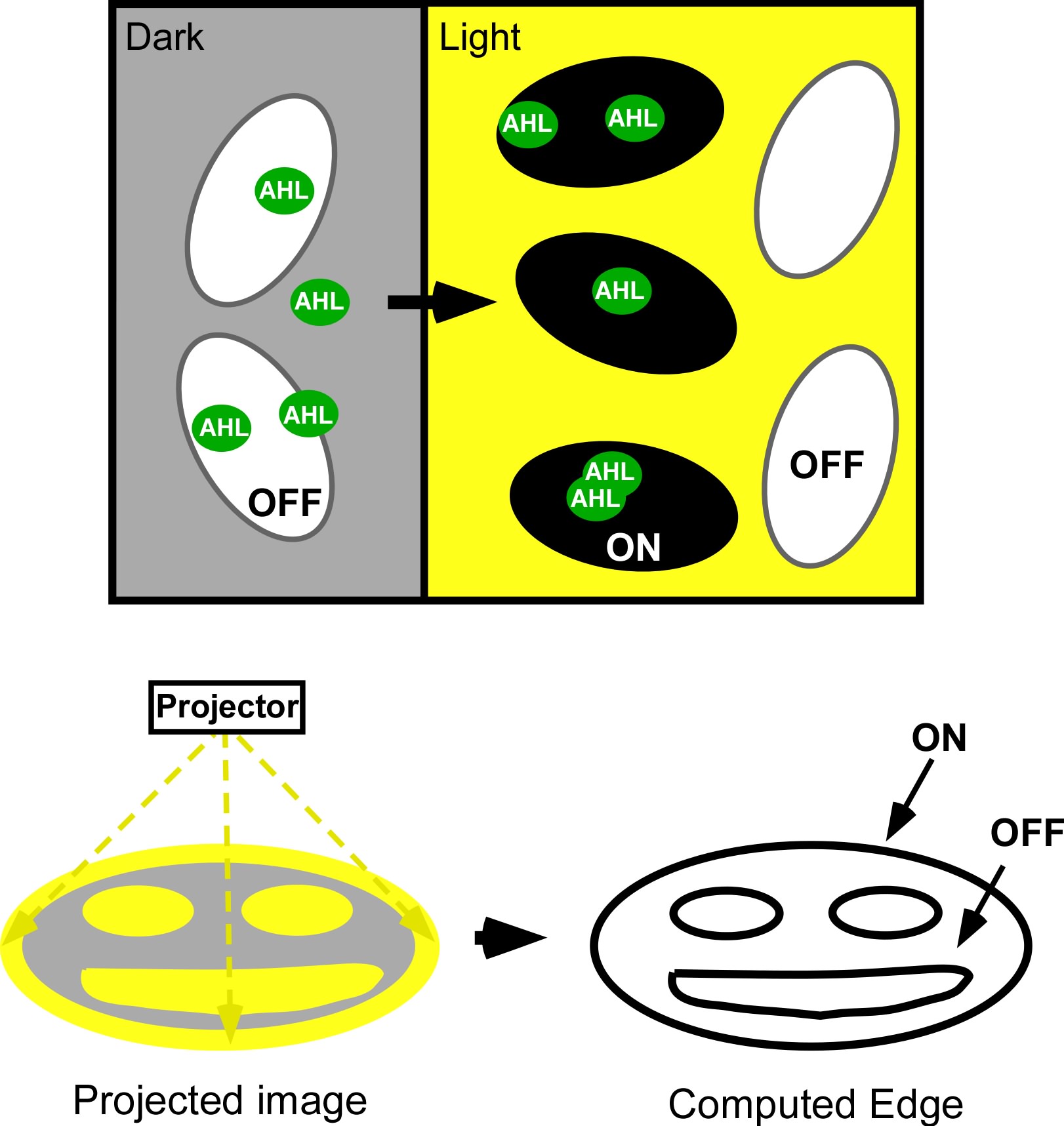
| + | <div>[[Image:Ed_logic.jpg|thumb|left|700px| Edge Detector Circuit and logic. (Left) The light sensing machinery from above has been black-boxed and the edge detection circuitry has been added downstream. Red light represses the expression of 2 genes; a biosynthetic gene for a membrane diffusible quorum sensing activator (AHL), and a dominant transcriptional repressor (cI). (Right) The output of the circuit (Z;Beta-galactosidase) is ON only in the presence of X (AHL) and the absence of Y (cI). This can only occur at the light/dark boundary.]]</div> | ||
| + | <div> | ||
| + | [[Image:jt_2.jpg|thumb|right|400px| (Top) Micro-scale edge detection. (Bottom) Macro-scale edge detection. An image is projected onto an agar plate of ''E.coli'' expressing the circuitry shown to the left. Cells residing in dark areas express AHL but are dominantly repressed by CI. Illuminated cells express neither AHL nor cI. If they neighbor a dark area, they will be activated by locally diffusing AHL. In this way, only cells at the light dark boundary express a reporter, and the edge is detected.]] | ||
| + | </div> | ||
| Line 51: | Line 56: | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 85: | Line 78: | ||
| + | ==Current Work: Photofabrication== | ||
| + | We have also developed methodologies that combine mask-directed lithography with multiphoton photo-crosslinking (MPP) to fabricate microstructures composed of cross-linked proteins with '''<font color="green">which living cells can directly interact</font>'''. This approach allows for the rapid prototyping of complex micro-containers with feature sizes as small a 200 microns. | ||
| + | |||
| + | Three dimensional structures can be built and used to trap and incubate small populations of cells, down to a single bacterium. Further, architectures can be proscribed to exploit cell motility and generate controlled, hydrodynamic microenvironments – a crucial step towards bio-powered pumps and mixers for microscale applications. These structures also have potential to be combined with engineered bacteria to control localized behavior of small bacterial populations or individuals. | ||
| + | '''<font color="red">Please attend our iGEM2006 talk to see MUCH more...</font>''' | ||
| + | [[Image:utexas2006-lithography.jpg|center|500px|]] | ||
| + | ==Future Work: Light Controlled Chemotaxis== | ||
| + | We would like to interface cellular motility with light detection. These bacteria could then be added to microstructures where bacteria power liquid flow through the structure in a light dependent manner. To accomplish this, we are currently cloning the CheR gene under the light-responsive OmpC promoter. Over expression of CheR results in methylation of the chemotaxis receptor which inhibits response to chemoattractants. | ||
| + | ==Favorite Parts== | ||
| + | *[http://partsregistry.org/Part:BBa_I15008 I15008]<br> | ||
| + | *[http://partsregistry.org/Part:BBa_I15009 I15009]<br> | ||
| + | *[http://partsregistry.org/Part:BBa_I15010 I15010]<br> | ||
| + | *[http://partsregistry.org/Part:BBa_I15022 I15022]<br> | ||
==Links== | ==Links== | ||
| + | [http://www.utexas.edu/ UT Austin] | ||
If you'd like to take your own pictures, check out the page on [http://openwetware.org/wiki/LightCannon how to build a light cannon] | If you'd like to take your own pictures, check out the page on [http://openwetware.org/wiki/LightCannon how to build a light cannon] | ||
Latest revision as of 09:41, 7 November 2006
The UT Austin team is:
- Aaron Chevalier
- Eric Davidson
- Bryan Kaehr
- [http://openwetware.org/wiki/User:LLavery Laura Lavery]
- Matt Levy
- [http://www.mine-control.com/ Zack Booth Simpson]
- [http://openwetware.org/wiki/User:Jeff_Tabor Jeff Tabor]
Advisors:
- [http://ellingtonlab.org/ Andy Ellington]
- [http://polaris.icmb.utexas.edu/home.html Edward Marcotte]
- [http://research.cm.utexas.edu/jshear Jason Shear]
Contents |
Previous work: Bacterial Photography
An obvious hurdle in the implementation of this system was genetically encoding light detection in the naturally blind organism, E.coli. To accomplish this, we used a synthetic part engineered in the Voigt lab. This part, Cph8, ([http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=5302 I15010]) is an engineered fusion between a cyanobacterial light sensing phytochrome (Cph1) and an E.coli transmembrane histidine kinase, (EnvZ). 660nm light causes an isomerization in the Cph1 domain of the chimera which inactivates the histidine kinase acitity of EnvZ. When EnvZ kinase activity is inhibited, a cascade which activates transcription from the OmpC promoter [http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=3910 R0082]) is also inhibited, while transcription from the OmpF promoter ([http://parts2.mit.edu/r/parts/partsdb/view.cgi?part_id=3915 R0084]) is activated.
We then demonstrated that when this system is expressed in E.coli, it is possible to transform each cell on an agar surface into a decision making pixel capable of reporting whether it is in the light or dark. The community of cells is therefore capable of genetically reproducing a light image.
This year, we have added to our previous work with Cph8 by further characterizing its response under varying conditions, including the effect of osmolarity and different cell strains. Further, it has been known that the chromophore responds in opposite ways to light around 660 versus 735nm. Previously, light around 660nm was used to repress signal transduction. We now show that light around 735nm can be used to further activate signal transduction. If both lights are used simultaneously, an increase in contrast can be achieved. We have also characterized the one-plasmid biobrick vector containing the genes for both Cph8 and the biosynthesis of the chromophore.
Current work: Edge detector
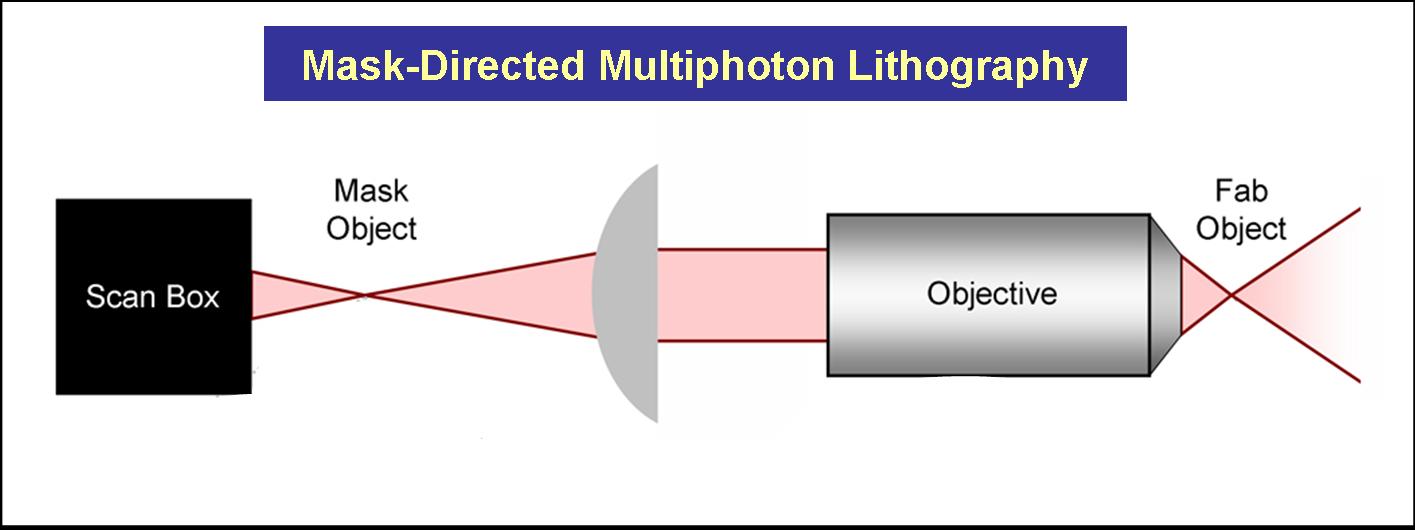
Current Work: Photofabrication
We have also developed methodologies that combine mask-directed lithography with multiphoton photo-crosslinking (MPP) to fabricate microstructures composed of cross-linked proteins with which living cells can directly interact. This approach allows for the rapid prototyping of complex micro-containers with feature sizes as small a 200 microns.
Three dimensional structures can be built and used to trap and incubate small populations of cells, down to a single bacterium. Further, architectures can be proscribed to exploit cell motility and generate controlled, hydrodynamic microenvironments – a crucial step towards bio-powered pumps and mixers for microscale applications. These structures also have potential to be combined with engineered bacteria to control localized behavior of small bacterial populations or individuals.
Please attend our iGEM2006 talk to see MUCH more...
Future Work: Light Controlled Chemotaxis
We would like to interface cellular motility with light detection. These bacteria could then be added to microstructures where bacteria power liquid flow through the structure in a light dependent manner. To accomplish this, we are currently cloning the CheR gene under the light-responsive OmpC promoter. Over expression of CheR results in methylation of the chemotaxis receptor which inhibits response to chemoattractants.
Favorite Parts
- [http://partsregistry.org/Part:BBa_I15008 I15008]
- [http://partsregistry.org/Part:BBa_I15009 I15009]
- [http://partsregistry.org/Part:BBa_I15010 I15010]
- [http://partsregistry.org/Part:BBa_I15022 I15022]
Links
[http://www.utexas.edu/ UT Austin]
If you'd like to take your own pictures, check out the page on [http://openwetware.org/wiki/LightCannon how to build a light cannon]