Mississippi State University 2006
From 2006.igem.org
(Difference between revisions)
| (59 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
[[Image:mstate2.jpg]]<br> | [[Image:mstate2.jpg]]<br> | ||
<h1>Welcome to [http://www.msstate.edu Mississippi State University!]</h1> | <h1>Welcome to [http://www.msstate.edu Mississippi State University!]</h1> | ||
| - | | width= | + | | width=500px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | |
[[Image:iGEM 2006 024.jpg]]<br> | [[Image:iGEM 2006 024.jpg]]<br> | ||
| - | + | From left to right: Teri Vaughn <sub>1</sub>, Scott Tran <sub>1</sub>, Lauren Beatty <sub>1</sub>, Robert Morris <sub>1</sub>, Victor Ho <sub>2</sub>, Dr. Din-Pow Ma <sub>2</sub>, Dr. Filip To <sub>1</sub><br> | |
| + | [http://www.abe.msstate.edu/Undergraduate/Biological/ <sub>1</sub> Biological Engineering Department] [http://www.msstate.edu/dept/biochemistry/ <sub>2</sub> Biochemistry Department] | ||
|} | |} | ||
| Line 20: | Line 21: | ||
: [mailto:reese@ece.msstate.edu Dr. Bob Reese] Electrical and Computer Engineering<br> | : [mailto:reese@ece.msstate.edu Dr. Bob Reese] Electrical and Computer Engineering<br> | ||
: [mailto:french@che.msstate.edu Dr. Tod French] Chemical Engineering<br> | : [mailto:french@che.msstate.edu Dr. Tod French] Chemical Engineering<br> | ||
| - | : [mailto:dm1@ra.msstate.edu Dr. Din-Pow Ma] | + | : [mailto:dm1@ra.msstate.edu Dr. Din-Pow Ma] Biochemistry<br> |
'''Students:''' | '''Students:''' | ||
: [mailto:tmv16@msstate.edu Teri Vaughn] Undergraduate, Senior, Biomedical Engineering<br> | : [mailto:tmv16@msstate.edu Teri Vaughn] Undergraduate, Senior, Biomedical Engineering<br> | ||
| + | : [mailto:cjh93@msstate.edu Courtney Harbin] Undergraduate, Senior, Biochemistry<br> | ||
: [mailto:leb120@msstate.edu Lauren Beatty] Undergraduate, Junior, Biomedical Engineering<br> | : [mailto:leb120@msstate.edu Lauren Beatty] Undergraduate, Junior, Biomedical Engineering<br> | ||
: [mailto:sct32@msstate.edu Scott Tran] Undergraduate, Junior, Biological Engineering<br> | : [mailto:sct32@msstate.edu Scott Tran] Undergraduate, Junior, Biological Engineering<br> | ||
| + | : [mailto:swp50@msstate.edu Sam Pote] Undergraduate, Freshman, Biological Engineering<br> | ||
| + | : [mailto:pbk8@msstate.edu Paul Kimbrough] Undergraduate, Junior, Biological Engineering<br> | ||
| + | : [mailto:jc359@msstate.edu Joseph Chen] Undergraduate, Junior, Biological Engineering<br> | ||
: [mailto:rmm72@msstate.edu Robert Morris] Grad student, Biological Engineering<br> | : [mailto:rmm72@msstate.edu Robert Morris] Grad student, Biological Engineering<br> | ||
: [mailto:mh277@msstate.edu Meng-Hsuan Ho] Grad student, Molecular Biology<br> | : [mailto:mh277@msstate.edu Meng-Hsuan Ho] Grad student, Molecular Biology<br> | ||
| Line 32: | Line 37: | ||
|width=325px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | | |width=325px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<h3>Introduction</h3> | <h3>Introduction</h3> | ||
| Line 53: | Line 43: | ||
* International Genetically Engineered Machine (iGEM) is a student-led competition to build the most innovative "machine" by synthetic biology. | * International Genetically Engineered Machine (iGEM) is a student-led competition to build the most innovative "machine" by synthetic biology. | ||
* Headquarters is located at the Massachusetts Institute of Technology. | * Headquarters is located at the Massachusetts Institute of Technology. | ||
| - | * In 2006, | + | * In 2006, 37 schools and over 400 students from around the world are participating in projects to construct biologically engineered systems. |
* Task of each team is to apply engineering methodology to design and develop a new biological system ("machine") through the use of existing and/or newly formed microscopic biological parts (termed BioBricks). | * Task of each team is to apply engineering methodology to design and develop a new biological system ("machine") through the use of existing and/or newly formed microscopic biological parts (termed BioBricks). | ||
* Type of the "machine" is chosen by each individual school participating, and the only criterion is that the "machine" be made entirely of the functional units of DNA called BioBricks. | * Type of the "machine" is chosen by each individual school participating, and the only criterion is that the "machine" be made entirely of the functional units of DNA called BioBricks. | ||
| Line 67: | Line 57: | ||
<h3>H<sub>2</sub> Reporter</h3> | <h3>H<sub>2</sub> Reporter</h3> | ||
| - | * Syngas is a renewable source of energy that has the potential to improve and relieve the energy crisis in the world today. Syngas has the capability to | + | * Syngas is a renewable source of energy that has the potential to improve and relieve the energy crisis in the world today. Syngas has the capability to yield products such as ethanol, bio-diesel, and bio-oil, which could replace gasoline and diesel as the major energy sources in the future. The problem, however, lies in the process of producing a high-quality syngas. In general, a high-quality syngas consists of low tar content and high levels of H<sub>2</sub> and CO. The high levels of H<sub>2</sub> and CO increase the combustibility and heating value of the syngas, making it more efficient and effective. |
* Our purpose for iGEM 2006 is to build a machine containing a reporter capable of detecting the presence of H<sub>2</sub> and producing YFP if H<sub>2</sub> is present. | * Our purpose for iGEM 2006 is to build a machine containing a reporter capable of detecting the presence of H<sub>2</sub> and producing YFP if H<sub>2</sub> is present. | ||
| - | * | + | *Our team chose to test the hybB promoter to see if it had hydrogen sensing capabilities. We also placed switches in the constructions in order to be able to control our detectors. For a reporter, we chose a YFP. |
| - | :[[Image: | + | |
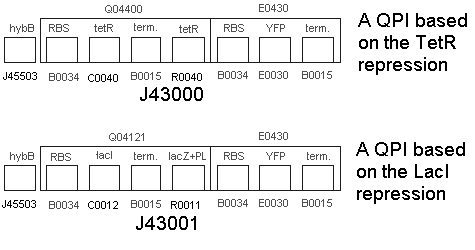
| + | * We built the following two constructs:<br> | ||
| + | :[[Image:untitled6.gif]] | ||
|} | |} | ||
| + | |||
| + | {| cellspacing="2px" cellpadding="0" border="0" style="padding: 0; width: 750px; color: #000000; background-color: #ffffff;" | ||
| + | | width=717px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | | ||
| + | |||
| + | <h3>Results</h3> | ||
| + | |||
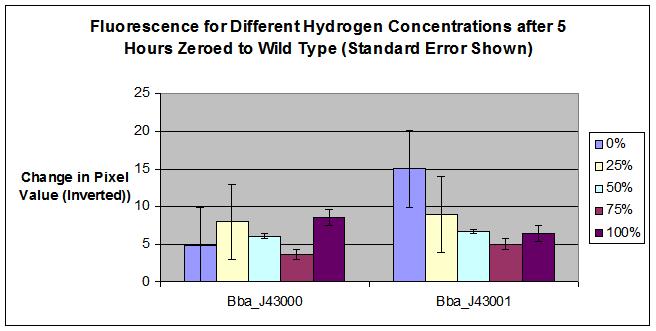
| + | :[[Image:MSUResultsErrorbars.JPG]] | ||
| + | |||
| + | * Part BBa_J43000 | ||
| + | |||
| + | * No Hydrogen, No tetR = fluorescence | ||
| + | * No Hydrogen, with tetR = Repressed Transcription at R0040 tetR, no fluorescence (except for some leakage). | ||
| + | * Hydrogen + tetR = start trqanscription at hybB, produce tetR, increased repression of operon at R0040 tetR. no fluorescence. | ||
| + | |||
| + | |||
| + | * Part BBa_J43001 | ||
| + | |||
| + | * No Hydrogen, No inducer = no fluorescence (except for a bit of leakage possibly). | ||
| + | * No Hydrogen, Inducer = Start Transcription at lacZ+PL, fluoresce. | ||
| + | * Hydrogen + Inducer = start transcription at hybB, produce lacI, competitive inhibition of operon at lacZ+PL. Less fluorescence transcribed. | ||
| + | |||
| + | |} | ||
| + | |||
| + | {| cellspacing="2px" cellpadding="0" border="0" style="padding: 0; width: 750px; color: #000000; background-color: #ffffff;" | ||
| + | | width=717px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | | ||
| + | |||
| + | <h3>Discussion</h3> | ||
| + | |||
| + | * Complications: | ||
| + | |||
| + | * BBa_J43000 was a failed part, we learned during completion of the construction and testing that there was a flaw in the design of the composite part. Although the hybB promoter does appear to detect hydrogen, the YFP is inhibited with the addition of tetR. Therefore, we cannot detect a change in fluorescence when hydrogen is added. | ||
| + | * For the BBa_J43001, calculated error provides a possible explaination for why 100% hydrogen concentration fluoresced more than 75% hydrogen concentration. More testing required to determine the 100% hydrogen result. | ||
| + | |||
| + | |||
| + | * Achievements: | ||
| + | |||
| + | * The results of our test agree with possible success for part BBa_J43001. More testing must be performed to further prove our part as a hydrogen detector. | ||
| + | * Our machine confirms the success of hybB, BBa_Q04121, and BBa_E0430 as a composite part | ||
| + | |||
| + | |||
| + | |} | ||
| + | |||
| + | {| cellspacing="2px" cellpadding="0" border="0" style="padding: 0; width: 750px; color: #000000; background-color: #ffffff;" | ||
| + | | width=717px style="padding: 5px; background-color: #ffffff; border: 2px solid #993300;" | | ||
| + | |||
| + | <h3>Accomplishments</h3> | ||
| + | |||
| + | * October 25- Gather results from final tests and analyze. | ||
| + | * October 24- Took pictures of vials for final tetsing. | ||
| + | * October 23- Performed gassing for final testing (Solid Media). | ||
| + | * October 22- Inoculation of E.coli + plasmid into solid media. | ||
| + | * October 17- Sent two constructions to the registry, and registered our parts on the website. | ||
| + | * October 5- Analyzed results of second test. | ||
| + | * October 4- Took pictures of vials for second testing. | ||
| + | * October 3- Performed gassing for second testing (Liquid Media). | ||
| + | * October 2- Inoculation of E.coli + plasmid into liquid media. | ||
| + | * September 29- Built testing station to be used with spectrometer. | ||
| + | * September 22- Analyzed results of first test. | ||
| + | * September 21- Took pictures of vials in first test. | ||
| + | * September 20- Performed gassing of first test (Solid Media). | ||
| + | * September 18- Inoculation of E.coli + plasmid into solid media. | ||
| + | * September 12-14- Gathered resources to begin testing. | ||
| + | * August 29-September 8- Searched for the instrumentation needed to do the testing. | ||
| + | * August 24- Digested plasmids to test the ligation of HybB (successful). | ||
| + | * August 22- Sequenced promoter for verification | ||
| + | * August 18- Group meeting with faculty, Publicity and preparation for Jamboree discussed. | ||
| + | * August 16- Planning for Jamboree with help from James. | ||
| + | * August 15- Group meeting with James our Ambassador. iGEM presentations given by James. | ||
| + | * August 14- Digested plasmids to test the ligation of HybB (unsuccessful). | ||
| + | * August 13- Isolated plasmids HybB-Q04121-E0430 and HybB-Q04400-E0430. | ||
| + | * August 9- Ligation of HybB promoter with the two constructions. | ||
| + | * August 7- Digested and purified the two constructions. | ||
| + | * August 3- Digested plasmids to confirm the constructions of Q04121-E0430 and Q04400-E0430. | ||
| + | * August 2- Isolated plasmids Q04121-E0430 and Q04400-E0430. | ||
| + | * July 31- Isolated and confirmed Q04400. Reconstructed Q04121-E0430. Constructed Q04400-E0430. | ||
| + | * July 28- Isolated and analyzed Q04121-E0430 (unsuccessful). | ||
| + | * July 27- Initial project information and pictures sent to Kirsten at Momentum. | ||
| + | * July 26- Ligased Q04121 and E0430 together and transformed into E. coli. Regrew Q04400 (first attempt at growth was unsuccessful). | ||
| + | * July 25- Performed polymerase chain reaction. | ||
| + | * July 21- Digested plasmid. | ||
| + | * July 19- Harvested cells and performed electrophoresis. | ||
| + | * July 18- Cultivated and innoculated colonies. | ||
| + | * July 17- Transformed DNA into E. coli. | ||
| + | * July 14- Removed chosen parts from DNA plates. Modified one part of design: E0430 substituted for E0432. | ||
| + | * July 13- Began lab work. Prepared agar plates. | ||
| + | * July 10- Student meeting, BioBrick parts chosen. | ||
| + | * July 6 - Group meeting with faculty, Steps to build machine defined and parameters discussed. | ||
| + | * June 8 - Group meeting with faculty, Project goals defined. | ||
Latest revision as of 19:33, 30 October 2006
The TeamFaculty Members:
Students:
|
Introduction
|
H2 Reporter
|
Results
|
Discussion
|
Accomplishments
|