Berkeley2006-RiboregulatorsMain
From 2006.igem.org
JCAnderson (Talk | contribs) (→) |
JCAnderson (Talk | contribs) (→) |
||
| (19 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
'''Within our addressable conjugation system, every cell contains a unique nucleic acid "address" sequence. This address is encoded within an RNA riboregulator "lock" and is used to control the bacterium's ability to communicate with other cells. It was imperative to develop high-gain riboregulators that can be rationally modified to construct many orthogonal lock/key pairs. Here we show the evolution of the original riboregulator design into a high-performance regulatory system.'''<br> | '''Within our addressable conjugation system, every cell contains a unique nucleic acid "address" sequence. This address is encoded within an RNA riboregulator "lock" and is used to control the bacterium's ability to communicate with other cells. It was imperative to develop high-gain riboregulators that can be rationally modified to construct many orthogonal lock/key pairs. Here we show the evolution of the original riboregulator design into a high-performance regulatory system.'''<br> | ||
=== === | === === | ||
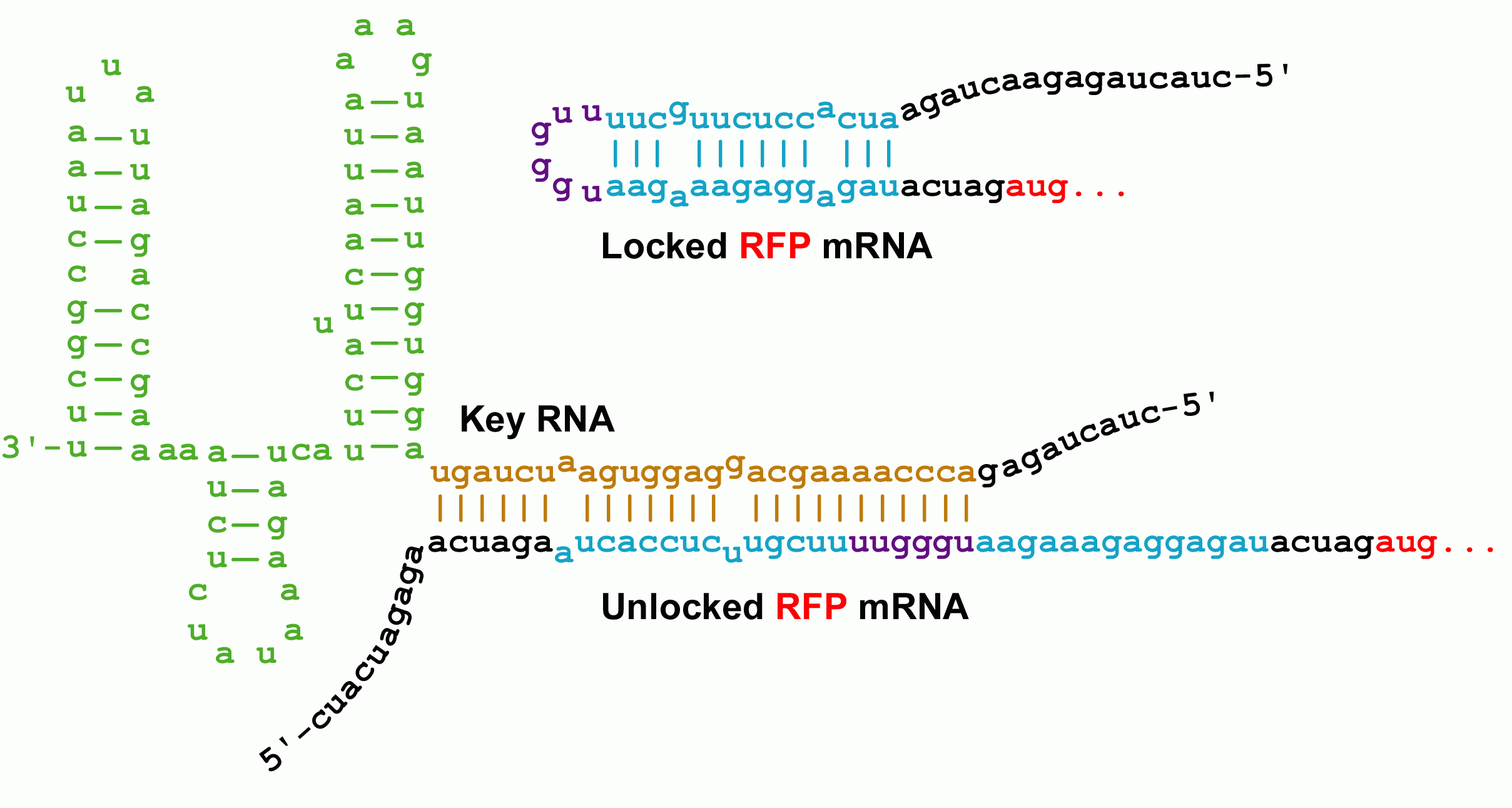
| - | + | Initially developed by [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=15208640&query_hl=1&itool=pubmed_docsum Collins and coworkers], riboregulators translationally control gene expression and are composed of two RNA parts--a lock and a key sequence. The lock sequence replaces the ribosome binding site (RBS) of the controlled gene. Within the lock, a short linker sequence connects the RBS to its own reverse complement. This results in a hairpin structure that occludes the RBS thereby preventing access to the ribosome. Shown below are the initial lock and key sequences for our studies <br> | |
[[Image:Berkeley2006Ribo2.GIF | 550px]]<br> | [[Image:Berkeley2006Ribo2.GIF | 550px]]<br> | ||
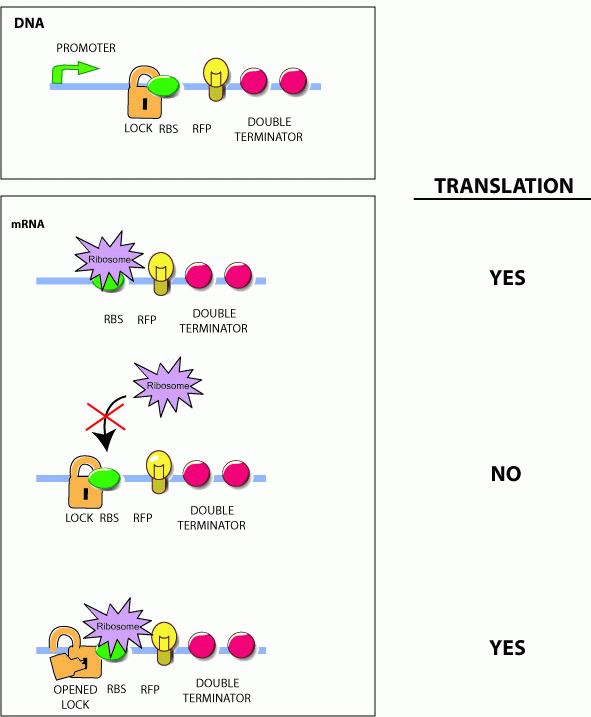
| - | Genes under the control of the lock show very little protein biosynthesis. The key sequence is expressed from a separate gene (''in trans'') and encodes a sequence complementary to the lock. ''The identity of this sequence is the address in our addressable conjugation system.'' When the key anneals to the lock sequence, the | + | Genes under the control of the lock show very little protein biosynthesis. The key sequence is expressed from a separate gene (''in trans'') and encodes a sequence complementary to the lock. ''The identity of this sequence is the address in our addressable conjugation system.'' When the key anneals to the lock sequence, the RBS becomes exposed permitting expression of the downstream gene. <br> |
[[Image:Berkeley2006Ribo1.GIF]]<br> | [[Image:Berkeley2006Ribo1.GIF]]<br> | ||
| + | |||
=== === | === === | ||
| - | The RNA sequence outside of the lock's | + | The RNA sequence outside of the lock's RBS binding region is of arbitrary sequence. However, this sequence must still match the key sequence to obtain unlocking. It should therefore be possible to construct millions of non-crossreactive variants of the riboregulator system.<br> |
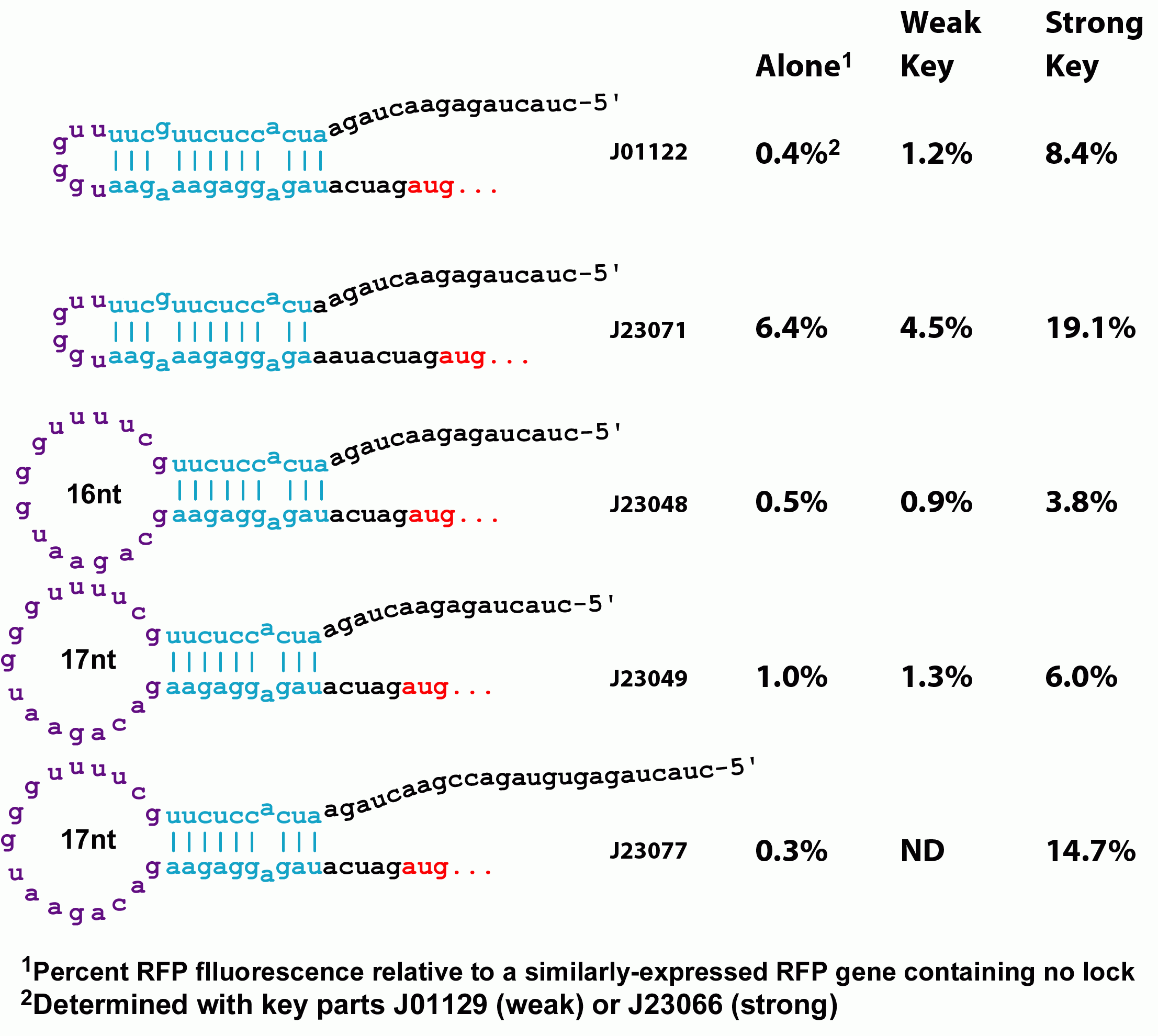
However, the original riboregulator design shows only a 1.7 fold unlocking of protein expression when the key is added. We therefore introduced a series of perturbations to the original design to determine the features of RNA locks and keys that would lead to a low background, high gain regulation system. | However, the original riboregulator design shows only a 1.7 fold unlocking of protein expression when the key is added. We therefore introduced a series of perturbations to the original design to determine the features of RNA locks and keys that would lead to a low background, high gain regulation system. | ||
| - | [[Image:Berkeley2006Ribo3.GIF]]<br> | + | [[Image:Berkeley2006Ribo3.GIF | 550px]]<br> |
=== === | === === | ||
| - | Variants of the lock with greater spacing between the RBS and its complementary sequence increases both the amount of background translation in the absense of the key as well as the degree of unlocking in the presence of the key, but does not do so proportionately--unlocking increases more than | + | Variants of the lock with greater spacing between the RBS and its complementary sequence increases both the amount of background translation in the absense of the key as well as the degree of unlocking in the presence of the key, but does not do so proportionately--unlocking increases more than the background. This could be a biophyiscal effect whereby a weaker lock is more amenable to the molecular motions that must occur to anneal with the lock. Alternatively, the extra spacing between the RBS and the key's annealing site might provide less steric hindrance with binding of the ribosome. Changing this spacing therefore could be used to tune the stringency of locking. Additionally, we constructed a stronger ribosome binding site variant. Our original key design is based on a non-optimal spacing between the RBS and the standard biobrick start codon. When this sequence is mutated to the optimal distance, both background translation and unlocking increase proportionately. |
| - | Next, we examined the interaction between the lock and the key. We constructed a variant of the lock with an extra 9 bp on the 5' end. This modification had no effect on the activity of the lock system. The extra sequence, however, could permit additional territory for the key to anneal. We are currently determining the optimal location for the addressing sequence within the lock. Two effects could improve unlocking here. First, the extra distance from the | + | Next, we examined the interaction between the lock and the key. We constructed a variant of the lock with an extra 9 bp on the 5' end. This modification had no effect on the activity of the lock system. The extra sequence, however, could permit additional territory for the key to anneal. We are currently determining the optimal location for the addressing sequence within the lock. Two effects could improve unlocking here. First, the extra distance from the RBS may make ribosome binding less hindered. Second, the 5' region of the lock is not occluded within the hairpin and is available for binding even when the lock is in the hairpin conformation. Therefore, unfolding of the hairpin is not a prerequisite for annealing of the key sequence and would be more favorable kinetically.<br> |
| + | [[Image:Berkeley2006Ribo4.GIF | 550px]]<br> | ||
| - | |||
=== === | === === | ||
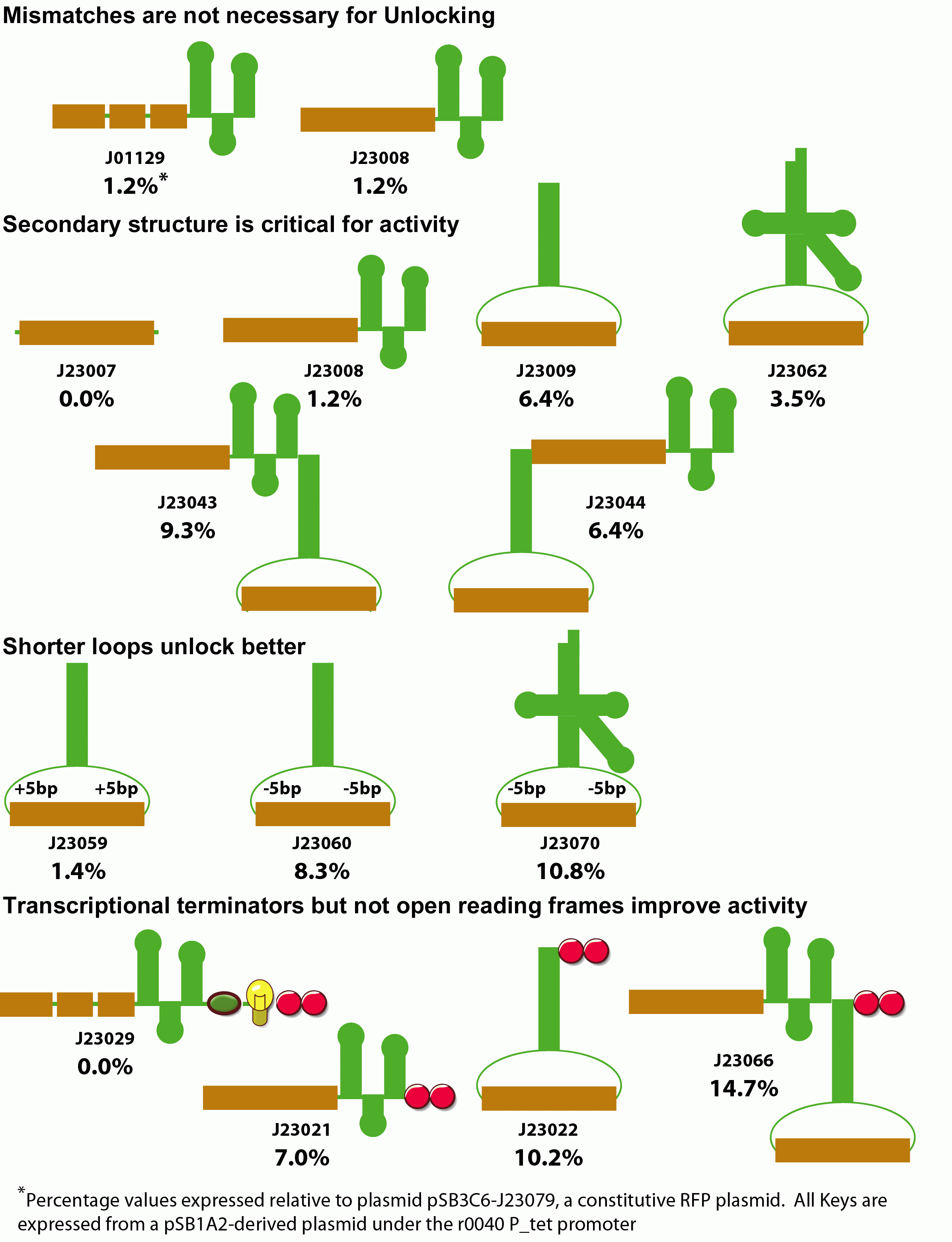
| - | + | We also examined modifications to the key that improve unlocking. Initially we examined whether mismatches between the lock and key annealing region were necessary for activity. Despite the potential susceptibility of the extended RNA duplex to RNAse degradation, perfect Watson-Crick base pairing within the annealing region had no effect on unlocking.<br> | |
| - | + | We next examined the role of secondary structure in riboregulator function. We made variants that deleted the hairpin sequence, moved the position of the annealing region to the loop of a large, very stable RNA hairpin, or the anticodon loop of a Ser2-derived tRNA. Additionally, we constructed fused dimers of these keys. Generally, increased secondary structure improves unlocking, presumably by protecting the RNA element from degradation.<br> | |
| - | We next examined the effect of fusing sequence to the | + | We next examined the effect of fusing sequence to the 3' ends of the key. When we placed an explicit terminator downstream of any of our functional keys, we observed a consistant 2-fold increase in unlocking activity. When we placed an open reading frame sequence and terminator downstream of the key, unlocking was entirely eliminated. These results suggest that shorter key transcripts lead to optimal unlocking. |
| + | Finally, we examined the spacing of the annealing region within the key's anticodon loop. We increased and decreased the spacing outside of the annealing region within the loop in both the hairpin and tRNA-like designs. Unexpectedly, the highest activity is observed with smaller loops for both the tRNA and hairpin designs. This most likely results from the periodicity of the RNA within the loop, or perhaps the rigidity imposed by a shorter loop in some way improves annealing with lock. | ||
| - | + | '''In summary, we have increased the gain of riboregulators from 2-fold to over 85-fold for our best lock/key combination. Additionally, by constructing keys with an internal rather than terminal annealing region, this system is more likely to be immune to variation in the upstream promoter than the original design.''' | |
---- | ---- | ||
Next section: [[Berkeley2006-ConjugationMain | Conjugation]] | Next section: [[Berkeley2006-ConjugationMain | Conjugation]] | ||
Latest revision as of 16:33, 30 October 2006
Within our addressable conjugation system, every cell contains a unique nucleic acid "address" sequence. This address is encoded within an RNA riboregulator "lock" and is used to control the bacterium's ability to communicate with other cells. It was imperative to develop high-gain riboregulators that can be rationally modified to construct many orthogonal lock/key pairs. Here we show the evolution of the original riboregulator design into a high-performance regulatory system.
Initially developed by [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=15208640&query_hl=1&itool=pubmed_docsum Collins and coworkers], riboregulators translationally control gene expression and are composed of two RNA parts--a lock and a key sequence. The lock sequence replaces the ribosome binding site (RBS) of the controlled gene. Within the lock, a short linker sequence connects the RBS to its own reverse complement. This results in a hairpin structure that occludes the RBS thereby preventing access to the ribosome. Shown below are the initial lock and key sequences for our studies
Genes under the control of the lock show very little protein biosynthesis. The key sequence is expressed from a separate gene (in trans) and encodes a sequence complementary to the lock. The identity of this sequence is the address in our addressable conjugation system. When the key anneals to the lock sequence, the RBS becomes exposed permitting expression of the downstream gene.
The RNA sequence outside of the lock's RBS binding region is of arbitrary sequence. However, this sequence must still match the key sequence to obtain unlocking. It should therefore be possible to construct millions of non-crossreactive variants of the riboregulator system.
However, the original riboregulator design shows only a 1.7 fold unlocking of protein expression when the key is added. We therefore introduced a series of perturbations to the original design to determine the features of RNA locks and keys that would lead to a low background, high gain regulation system.
Variants of the lock with greater spacing between the RBS and its complementary sequence increases both the amount of background translation in the absense of the key as well as the degree of unlocking in the presence of the key, but does not do so proportionately--unlocking increases more than the background. This could be a biophyiscal effect whereby a weaker lock is more amenable to the molecular motions that must occur to anneal with the lock. Alternatively, the extra spacing between the RBS and the key's annealing site might provide less steric hindrance with binding of the ribosome. Changing this spacing therefore could be used to tune the stringency of locking. Additionally, we constructed a stronger ribosome binding site variant. Our original key design is based on a non-optimal spacing between the RBS and the standard biobrick start codon. When this sequence is mutated to the optimal distance, both background translation and unlocking increase proportionately.
Next, we examined the interaction between the lock and the key. We constructed a variant of the lock with an extra 9 bp on the 5' end. This modification had no effect on the activity of the lock system. The extra sequence, however, could permit additional territory for the key to anneal. We are currently determining the optimal location for the addressing sequence within the lock. Two effects could improve unlocking here. First, the extra distance from the RBS may make ribosome binding less hindered. Second, the 5' region of the lock is not occluded within the hairpin and is available for binding even when the lock is in the hairpin conformation. Therefore, unfolding of the hairpin is not a prerequisite for annealing of the key sequence and would be more favorable kinetically.
We also examined modifications to the key that improve unlocking. Initially we examined whether mismatches between the lock and key annealing region were necessary for activity. Despite the potential susceptibility of the extended RNA duplex to RNAse degradation, perfect Watson-Crick base pairing within the annealing region had no effect on unlocking.
We next examined the role of secondary structure in riboregulator function. We made variants that deleted the hairpin sequence, moved the position of the annealing region to the loop of a large, very stable RNA hairpin, or the anticodon loop of a Ser2-derived tRNA. Additionally, we constructed fused dimers of these keys. Generally, increased secondary structure improves unlocking, presumably by protecting the RNA element from degradation.
We next examined the effect of fusing sequence to the 3' ends of the key. When we placed an explicit terminator downstream of any of our functional keys, we observed a consistant 2-fold increase in unlocking activity. When we placed an open reading frame sequence and terminator downstream of the key, unlocking was entirely eliminated. These results suggest that shorter key transcripts lead to optimal unlocking.
Finally, we examined the spacing of the annealing region within the key's anticodon loop. We increased and decreased the spacing outside of the annealing region within the loop in both the hairpin and tRNA-like designs. Unexpectedly, the highest activity is observed with smaller loops for both the tRNA and hairpin designs. This most likely results from the periodicity of the RNA within the loop, or perhaps the rigidity imposed by a shorter loop in some way improves annealing with lock.
In summary, we have increased the gain of riboregulators from 2-fold to over 85-fold for our best lock/key combination. Additionally, by constructing keys with an internal rather than terminal annealing region, this system is more likely to be immune to variation in the upstream promoter than the original design.
Next section: Conjugation