Berkeley2006-ConjugationMain
From 2006.igem.org
JCAnderson (Talk | contribs) |
JCAnderson (Talk | contribs) |
||
| (10 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
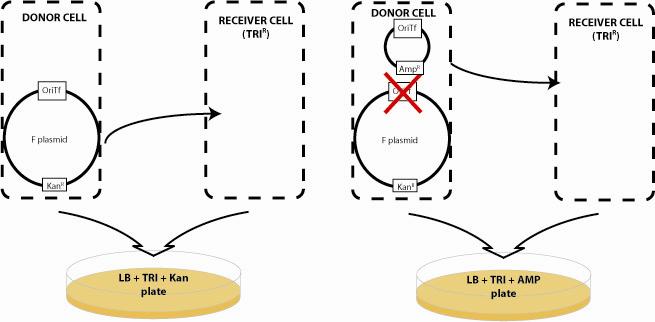
| - | Bacterial conjugation provides the communication system for our networks. Based on the natural F and RP4 plasmids, DNA "messages" can be sent from a donor cell to a recipient cell based on conjugation when the two cell types are mixed in liquid media or on a surface. Natural conjugative plasmids are large 60-100kb, circular plasmids that encode all the genes necessary for plasmid transfer including pili for bacteria-bacteria interactions, and genes that prevent superinfection of F and RP4-containing cells with secondary messages. Although F-type cells cannot send send plasmids to one another, F plasmid cells can send plasmids to R-type cells and vice versa. A critical component of transfer is the origin of transfer (oriT). This sequence within the conjugative plasmid initiates rolling circle amplification and transfer of the plasmid to the recipient cell. In our system, we have deleted the oriT region of the RP4 and F plasmid by the methods of [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=10829079&query_hl=3&itool=pubmed_docsum Wanner] such that these plasmids can no longer be transferred. | + | Bacterial conjugation provides the communication system for our networks. Based on the natural F and RP4 plasmids, DNA "messages" can be sent from a donor cell to a recipient cell based on conjugation when the two cell types are mixed in liquid media or on a surface. Natural conjugative plasmids are large 60-100kb, circular plasmids that encode all the genes necessary for plasmid transfer including pili for bacteria-bacteria interactions, and genes that prevent superinfection of F and RP4-containing cells with secondary messages. Although F-type cells cannot send send plasmids to one another, F plasmid cells can send plasmids to R-type cells and vice versa. A critical component of transfer is the origin of transfer (oriT). This sequence within the conjugative plasmid initiates rolling circle amplification and transfer of the plasmid to the recipient cell. In our system, we have deleted the oriT region of the RP4 and F plasmid by the methods of [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=10829079&query_hl=3&itool=pubmed_docsum Wanner] such that these plasmids can no longer be transferred. The Biobricked oriT parts are then inserted into small "message" plasmids and can be transferred to recipient cells by cells expressing a cognate F or R transfer system. |
[[Image:Berkeley2006_Conjugation1.jpg]] | [[Image:Berkeley2006_Conjugation1.jpg]] | ||
=== === | === === | ||
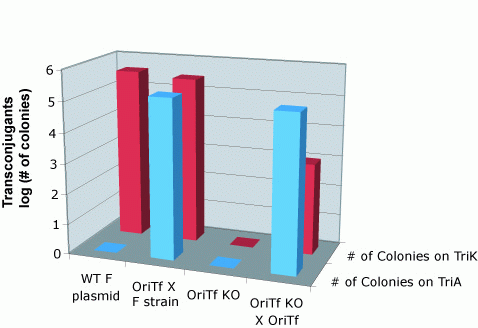
| - | To quantify the efficiency of transfer, we exploit antibiotic markers present in | + | To quantify the efficiency of transfer, we exploit antibiotic markers present in the F and R plasmid (Kanamycin), markers present on the message plasmid (Ampicillin), and present in the recipient genome (Trimethoprim). When non-mated cells are spread on plates containing trimethoprim and ampicillin, or trimethoprim and kanamycin, no colonies form. After mating, recipient cells that have recieved the transfer machinery can survive on trimethoprim and kanamycin plates. Cells that receive the message plasmid can grow on trimethoprim and ampicillin plates. By counting the number of colonies formed on each plate, we can determine the transfer efficiency.<br> |
| + | As seen below, both the R and F transfer systems (RLambda and J23055) send the message plasmid (pSB1A2-J01003 or pSB1A2-J01064) to recipient cells but not the transfer machinery.<br> | ||
| + | '''Transfer of message plasmids by the F system''' | ||
| - | |||
| - | |||
| - | |||
[[Image:Berkeley2006_Conjugation2.GIF]] | [[Image:Berkeley2006_Conjugation2.GIF]] | ||
| - | + | '''Transfer of messages by the R system'''<br> | |
| - | '''R | + | |
| - | + | ||
[[Image:Conjugation3.jpg]] | [[Image:Conjugation3.jpg]] | ||
| - | + | === === | |
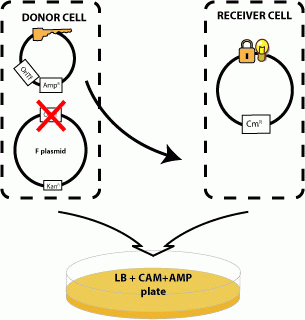
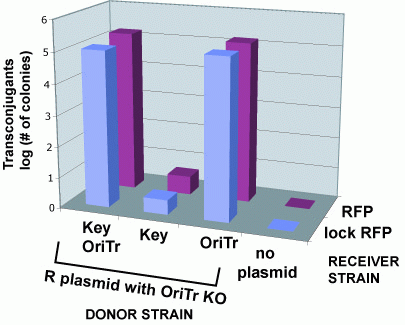
| + | We next examined whether sending a message with a key could unlock the riboregulator present in a recipient cell. To test this, we contructed plasmid pJ23006-J23086 with the R-type oriT and a constitutively-expressed key and inserted it into cells containing the R transfer plasmid. We mated these donors with recipients containing a locked RFP gene (pSB3C6-J23077). Only donors containing both the tranfer origin and the key are able to unlock the recipients RFP gene. | ||
'''Cell Sends "Key" to Recipient Cell which Unlocks Message''' | '''Cell Sends "Key" to Recipient Cell which Unlocks Message''' | ||
| Line 26: | Line 24: | ||
[[Image:Berkeley2006_Conjugation5.GIF]] | [[Image:Berkeley2006_Conjugation5.GIF]] | ||
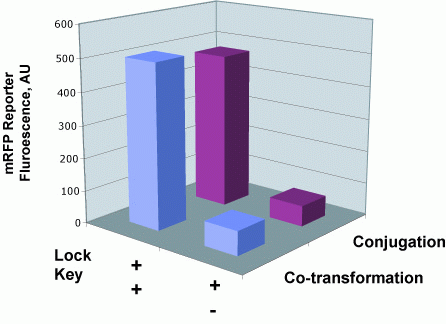
| + | To determine whether riboregulator locks and keys function normally upon conjugative transfer, we quantified the fluoresence of recipient cells that had received the key by conjugation and compared those values to cells that recieved the key sequence by normal transformation. | ||
'''Conjugation Does Not Impede Riboregulator Function''' | '''Conjugation Does Not Impede Riboregulator Function''' | ||
[[Image:Berkeley2006_Conjugation6.GIF]] | [[Image:Berkeley2006_Conjugation6.GIF]] | ||
| + | |||
| + | '''These experiments demonstrate the basic components of addressable conjugation. We have controlled the transfer of message plasmids and shown they function normally to activate cognate locked gene products.''' | ||
---- | ---- | ||
| - | Next Section: [[Berkeley2006-PromoterMain | | + | Next Section: [[Berkeley2006-PromoterMain | Controlling Message Sending and Receiving]] |
Latest revision as of 17:16, 30 October 2006
Bacterial conjugation provides the communication system for our networks. Based on the natural F and RP4 plasmids, DNA "messages" can be sent from a donor cell to a recipient cell based on conjugation when the two cell types are mixed in liquid media or on a surface. Natural conjugative plasmids are large 60-100kb, circular plasmids that encode all the genes necessary for plasmid transfer including pili for bacteria-bacteria interactions, and genes that prevent superinfection of F and RP4-containing cells with secondary messages. Although F-type cells cannot send send plasmids to one another, F plasmid cells can send plasmids to R-type cells and vice versa. A critical component of transfer is the origin of transfer (oriT). This sequence within the conjugative plasmid initiates rolling circle amplification and transfer of the plasmid to the recipient cell. In our system, we have deleted the oriT region of the RP4 and F plasmid by the methods of [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=10829079&query_hl=3&itool=pubmed_docsum Wanner] such that these plasmids can no longer be transferred. The Biobricked oriT parts are then inserted into small "message" plasmids and can be transferred to recipient cells by cells expressing a cognate F or R transfer system.
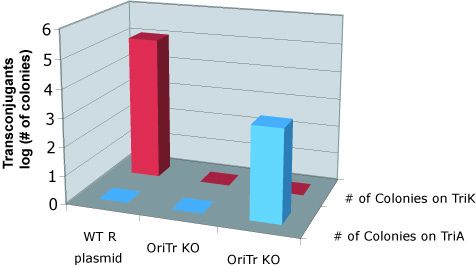
To quantify the efficiency of transfer, we exploit antibiotic markers present in the F and R plasmid (Kanamycin), markers present on the message plasmid (Ampicillin), and present in the recipient genome (Trimethoprim). When non-mated cells are spread on plates containing trimethoprim and ampicillin, or trimethoprim and kanamycin, no colonies form. After mating, recipient cells that have recieved the transfer machinery can survive on trimethoprim and kanamycin plates. Cells that receive the message plasmid can grow on trimethoprim and ampicillin plates. By counting the number of colonies formed on each plate, we can determine the transfer efficiency.
As seen below, both the R and F transfer systems (RLambda and J23055) send the message plasmid (pSB1A2-J01003 or pSB1A2-J01064) to recipient cells but not the transfer machinery.
Transfer of message plasmids by the F system
Transfer of messages by the R system
We next examined whether sending a message with a key could unlock the riboregulator present in a recipient cell. To test this, we contructed plasmid pJ23006-J23086 with the R-type oriT and a constitutively-expressed key and inserted it into cells containing the R transfer plasmid. We mated these donors with recipients containing a locked RFP gene (pSB3C6-J23077). Only donors containing both the tranfer origin and the key are able to unlock the recipients RFP gene.
Cell Sends "Key" to Recipient Cell which Unlocks Message
Riboregulator's Don't Effect Conjugation Efficiency
To determine whether riboregulator locks and keys function normally upon conjugative transfer, we quantified the fluoresence of recipient cells that had received the key by conjugation and compared those values to cells that recieved the key sequence by normal transformation.
Conjugation Does Not Impede Riboregulator Function
These experiments demonstrate the basic components of addressable conjugation. We have controlled the transfer of message plasmids and shown they function normally to activate cognate locked gene products.
Next Section: Controlling Message Sending and Receiving