4-State Device
From 2006.igem.org
(→Selected System) |
(temporary save of 4SD) |
||
| Line 3: | Line 3: | ||
= Purpose = | = Purpose = | ||
| - | The 4-State Device ( | + | The 4-State Device (4SD) uses two inputs to sequentially switch through its four states. The four states are used to keep track of changes of the inputs. One state is connected to the output. |
= Design = | = Design = | ||
| Line 9: | Line 9: | ||
== Suitable Strategies == | == Suitable Strategies == | ||
| - | For realizing the design we need one repressor for each state. For this purpose there are several possibilities that can be considered: | + | For realizing the design we need one repressor for each state. For this purpose there are several possibilities that can be considered: |
| - | * Silencing approach (antisense RNAs, short hairpin RNAs (shRNAs)) to reduce the pool of translatable mRNAs | + | |
| - | * Existing Repressor-Operator (for example TetR-TetO) | + | * Silencing approach (antisense RNAs, short hairpin RNAs (shRNAs)) to reduce the pool of translatable mRNAs |
| + | |||
| + | * Existing Repressor-Operator (for example TetR-TetO) | ||
| + | |||
* Designing new repressors (for example Zinc Finger Proteins) | * Designing new repressors (for example Zinc Finger Proteins) | ||
== Selected System == | == Selected System == | ||
| - | Considering the modularity as the first criterium for the selection of the system, we considered that the Zinc Finger Protein approach would be the optimal. Indeed, the operators could be as short as 9 nucleotide long and the specificity can be easily increased by either increasing the number of fingers in the ZFP or by coupling ZFP together via Leucine Zippers | + | Considering the modularity as the first criterium for the selection of the system, we considered that the Zinc Finger Protein approach would be the optimal. Indeed, the operators could be as short as 9 nucleotide long and the specificity can be easily increased by either increasing the number of fingers in the ZFP or by coupling ZFP together via Leucine Zippers. If were to use known repressor such as tetR, we would have to modify the promoter regions of the lambda-system, Pr and Prm. Although a valid approach it is dropped, due to increased workload, cost and lack of modularity |
| - | We chose the approach use design new repressors using zinc finger domains. Furthermore, we put the binding sites for the repressors directly after start of transcription and before the ribosome binding site to prevent RNA polymerase to transcribe the gene. This would allow us to put any promotor in from of the binding site and allowing a very high degree of modularity. | + | We chose the approach to use design new repressors using zinc finger domains. Furthermore, we put the binding sites for the repressors directly after start of transcription and before the ribosome binding site to prevent RNA polymerase to transcribe the gene. This would allow us to put any promotor in from of the binding site and allowing a very high degree of modularity. |
| - | + | If we want to use the possibility to use several 4SD connected to each order in order to count n^2, then for every new 4 state device four new repressors are needed. With zinc finger domains the construction of any binding site is possible and we will not run out of repressor. | |
| - | + | ||
| - | + | ||
= Actual Implementation = | = Actual Implementation = | ||
| Line 29: | Line 30: | ||
In the figure below is a parts-view of the device with PoPS interfaces (i/o). | In the figure below is a parts-view of the device with PoPS interfaces (i/o). | ||
| - | |||
==== Basic Functionality ==== | ==== Basic Functionality ==== | ||
The 4-State Device uses two inputs to sequentially switch through its four states. Note that this behaviour can be observed abstractly in the concept section. | The 4-State Device uses two inputs to sequentially switch through its four states. Note that this behaviour can be observed abstractly in the concept section. | ||
| - | To achieve such behaviour in our system, we use four interconnected gates. In the following, we call them ''NOR gates''. Each of our NOR gates has three inputs. All of them have to be low for the output to be high. | + | To achieve such behaviour in our system, we use four interconnected gates. In the following, we call them ''NOR gates''. Each of our NOR gates has three inputs. All of them have to be low for the output to be high. |
===== Electrical NOR gate ===== | ===== Electrical NOR gate ===== | ||
A NOR gate is a very common part in electrical engineering. | A NOR gate is a very common part in electrical engineering. | ||
| - | + | ||
| - | + | pic of NOR gate | |
| - | + | ||
| - | + | ||
| - | + | ||
===== Biological NOR gate ===== | ===== Biological NOR gate ===== | ||
Biologically, a NOR gate can be implemented through a promoter with high basal activity that is repressed by three effectors. If there is at least one of the three repressors, transcription is inhibited. | Biologically, a NOR gate can be implemented through a promoter with high basal activity that is repressed by three effectors. If there is at least one of the three repressors, transcription is inhibited. | ||
| - | + | Each NOR gates, expresses a repressor protein. (Note that such a biological NOR gate has no POPs interfaces) | |
biological picture of NOR gate | biological picture of NOR gate | ||
| Line 56: | Line 54: | ||
parts diagram | parts diagram | ||
| - | + | In the design of the Event Processing Device, input 1 and input 2 have opposite activity, meaning that either R1/R3 or R2/R4 is active. Furthermore, since R1 and R3 (respectively R2 and R4) are repressing each other, only one of the two is active. Therefore, in a stable situation, only 1 of the 4 repressor proteins is expressed. | |
Let us assume that R1 is being expressed. Input 2 must then be low, and therefore input 1 high. This situation is stable and remains until there is a change in the inputs. Now, if input 1 decreases, and input 2 increases, the expression of R1 will come to a halt. Since input 1 is now low, either R2 or R4 will be expressed. At this stage, R1 is still present in relatively high concentration and by repressing R4, it tips the balance in favor of R2, leading to a new stable state in which only R2 is expressed. | Let us assume that R1 is being expressed. Input 2 must then be low, and therefore input 1 high. This situation is stable and remains until there is a change in the inputs. Now, if input 1 decreases, and input 2 increases, the expression of R1 will come to a halt. Since input 1 is now low, either R2 or R4 will be expressed. At this stage, R1 is still present in relatively high concentration and by repressing R4, it tips the balance in favor of R2, leading to a new stable state in which only R2 is expressed. | ||
| Line 62: | Line 60: | ||
Note that electrical engineers call such a device a "J-K flip flop". It can also be seen as a combination of two toggle switches ([[Atkinson03]]), each being able to store one bit. | Note that electrical engineers call such a device a "J-K flip flop". It can also be seen as a combination of two toggle switches ([[Atkinson03]]), each being able to store one bit. | ||
| - | + | === Biological Details === | |
In the design of the repressor protein we use the following domains: | In the design of the repressor protein we use the following domains: | ||
| - | Zinc Finger (ZF) | + | - ''Zinc Finger (ZF)'' |
| - | DNA- | + | A Zinc Finger (ZF) [http://en.wikipedia.org/wiki/Zinc_finger] is a protein domain [http://en.wikipedia.org/wiki/Protein_domain] that binds to three base pairs of double stranded DNA. A Zinc Finger Protein (ZFP) consists of one or several zinc finger domains. Many protein-DNA interaction for ZF domains and triplet of base pairs have been described, therefore making it possible to to construct artificial transcription factors by combining ZF domains in a modular fashion. |
| - | NTD | + | - ''NTD'' |
The N-terminal polymerase domain from NusA. This protein has a RNA polymerase halting properties. | The N-terminal polymerase domain from NusA. This protein has a RNA polymerase halting properties. | ||
| - | ERD | + | - ''ERD'' |
ERF repressor domain. a repressor domain in eukaryotes. | ERF repressor domain. a repressor domain in eukaryotes. | ||
| - | LZ | + | -''LZ'' |
| - | + | ||
Leucine zipper. For dimerization. | Leucine zipper. For dimerization. | ||
| - | |||
| Line 92: | Line 88: | ||
| - | + | The different designs of repressors | |
| + | |||
| + | BioBrick Part Number of ZF domains LZ domain TF domain | ||
| + | |||
| + | BBa_J05101 3 - - | ||
| + | |||
| + | BBa_J05108 3* CREBH - | ||
| + | |||
| + | BBa_J05109 3* ATF6 - | ||
| + | |||
| + | BBa_J05110 3* YAP7 - | ||
| + | |||
| + | BBa_J05111 3* cMaf - | ||
| + | |||
| + | BBa_J05112 3 - ERD | ||
| + | |||
| + | BBa_J05113 3* ATF6 KRAB | ||
| + | |||
| + | BBa_J05114 3* ATF6 NTD | ||
| + | |||
| + | BBa_J05115 6 - - | ||
| + | |||
| + | |||
| + | |||
| + | ==== BioBrick parts ==== | ||
| + | |||
| + | |||
| + | BBa_J05040 4-State Device | ||
| + | |||
| + | BBa_J05108 Repressor R1-CREBH | ||
| + | |||
| + | BBa_J05109 Repressor R3-ATF6 | ||
| + | |||
| + | BBa_J05110 Repressor R2-YAP7 | ||
| + | |||
| + | BBa_J05111 Repressor R4-cMaf | ||
| + | |||
| + | BBa_J05215 Regulator for R1-CREBH | ||
| + | |||
| + | BBa_J05216 Regulator for R3-ATF6 | ||
| + | |||
| + | BBa_J05217 Regulator for R2-YAP7 | ||
| + | |||
| + | BBa_J05218 Regulator for R4-cMaf | ||
| + | |||
| + | BBa_J05311 R1 state | ||
| + | |||
| + | BBa_J05312 R3 state | ||
| + | |||
| + | BBa_J05313 R2 state | ||
| + | |||
| + | BBa_J05314 R4 state | ||
| + | |||
| + | |||
| + | |||
| + | BBa_J05000 Zinc Finger Protein Tester | ||
| + | |||
| + | BBa_J05112 Repressor R3-e2+ERD | ||
| + | |||
| + | BBa_J05113 Repressor R3-ATF6+KRAB | ||
| + | |||
| + | BBa_J05114 Repressor R3-ATF6+NTD | ||
| + | |||
| + | BBa_J05101 Repressor R3-e2 | ||
| + | |||
| + | BBa_J05115 Repressor ZF-2*e2 | ||
| + | |||
| + | BBa_J05221 Tripple Binding Site for R3-ATF6 | ||
| + | |||
| + | BBa_J05222 ZF-2*e2 Binding Site | ||
| - | + | BBa_J05216 Regulator for R3-ATF6 | |
| - | |||
| - | |||
| - | |||
| - | |||
| Line 108: | Line 169: | ||
|+ Ordered Sequences | |+ Ordered Sequences | ||
| - | ! Nickname !! Length | + | ! Nickname !! Length !! SacI !! KpnI !! NheI !! EcoRI !! XbaI !! SpeI !! PstI |
|- | |- | ||
| - | | BBa_J05215+BBa_J05108 || | + | | BBa_J05215+BBa_J05108 || 603 || 1 || 91 || 598 || 7, 97 || 22, 112 || 71, 578 || 85, 592 |
|- | |- | ||
| - | | BBa_J05216+BBa_J05109 || | + | | BBa_J05216+BBa_J05109 || 582 || 1 || 91 || 577 || 7, 97 || 22, 112 || 71, 557 || 85, 571 |
|- | |- | ||
| - | | BBa_J05217+BBa_J05110 || | + | | BBa_J05217+BBa_J05110 || 609 || 1 || 91 || 604 || 7, 97 || 22, 112 || 71, 584 || 85, 598 |
|- | |- | ||
| - | | BBa_J05218+BBa_J05111 || | + | | BBa_J05218+BBa_J05111 || 600 || 1 || 91 || 595 || 7, 97 || 22, 112 || 71, 575 || 85, 589 |
|- | |- | ||
| - | | BBa_J05221+BBa_J05101 || | + | | BBa_J05221+BBa_J05101 || 498 || 1 || 112 || 493 || 7, 118 || 22, 133 || 92, 473 || 106, 487 |
|- | |- | ||
| - | | BBa_J05114 || | + | | BBa_J05114 || 854 || 1 || 849 || - || 7 || 22 || 829 || 843 |
|- | |- | ||
| - | | BBa_J05112 || | + | | BBa_J05112 || 524 || 1 || 519 || - || 7 || 22 || 499 || 513 |
|- | |- | ||
| - | | BBa_J05222+BBa_J05115 || | + | | BBa_J05222+BBa_J05115 || 676 || 1 || 87 || 671 || 7, 93 || 22, 108 || 67, 651 || 81, 665 |
|} | |} | ||
| - | + | The provider of the synthesized DNA used for the zinc fingerers has a lower cost limit of $500. To reduce cost per base pair for short sequences we put the short sequences together with a long sequence with restriction sites between them. the trick we use to reduce cost, by having an extra step of cloning. | |
binding of ZFP. describe choice of binding sites. Describe the function of RNA pol II. emphasize: modularity! | binding of ZFP. describe choice of binding sites. Describe the function of RNA pol II. emphasize: modularity! | ||
| Line 145: | Line 206: | ||
{| border="1" cellpadding="2" | {| border="1" cellpadding="2" | ||
| - | ! Part !! Binding Site Seq !! # exact matches !! Hamming Dist 1 !! Hamming Dist 2 !! Hamming Dist 3 | + | ! Part !! Binding Site Seq !! est Kd [nM] !! # exact matches !! Hamming Dist 1 !! Hamming Dist 2 !! Hamming Dist 3 |
|- | |- | ||
| - | | BBa_J05100 || ggaggggac || | + | | BBa_J05100 || ggaggggac || 4 || 5 || 267 || 6196 || 65733 |
|- | |- | ||
| - | | BBa_J05102 || ggaggcggg || | + | | BBa_J05102 || ggaggcggg || 30 || 17 || 799 || 12589 || 95996 |
|- | |- | ||
| - | | BBa_J05101 || gggggcgag || | + | | BBa_J05101 || gggggcgag || 3 || 15 || 634 || 10173 || 82956 |
|- | |- | ||
| - | | BBa_J05103 || ggggccgga || | + | | BBa_J05103 || ggggccgga || 45 || 44 || 848 || 11317 || 91422 |
|- | |- | ||
| - | | BBa_J05108 || gtcccctccggaggggac || 0 || 0 || 0 | + | | BBa_J05108 || gtcccctccggaggggac || N/A || 0 || 0 || 0 || 1 |
|- | |- | ||
| - | | BBa_J05109 || ctcgcccccgggggcgag || 0 || 0 || 0 | + | | BBa_J05109 || ctcgcccccgggggcgag || N/A || 0 || 0 || 0 || 2 |
|- | |- | ||
| - | | BBa_J05110 || cccgcctccggaggcggg || 0 || 0 || 0 | + | | BBa_J05110 || cccgcctccggaggcggg || N/A || 0 || 0 || 0 || 4 |
|- | |- | ||
| - | | BBa_J05111 || tccggccccggggccgga || 0 || 0 || 0 | + | | BBa_J05111 || tccggccccggggccgga || N/A || 0 || 0 || 0 || 6 |
|- | |- | ||
| - | | BBa_J05115 || gggggcgaggggggcgag || 0 || 0 || 0 | + | | BBa_J05115 || gggggcgaggggggcgag || N/A || 0 || 0 || 0 || 3 |
|} | |} | ||
Revision as of 16:00, 31 October 2005
Back to the ETH Zurich main page.
Contents |
Purpose
The 4-State Device (4SD) uses two inputs to sequentially switch through its four states. The four states are used to keep track of changes of the inputs. One state is connected to the output.
Design
Suitable Strategies
For realizing the design we need one repressor for each state. For this purpose there are several possibilities that can be considered:
- Silencing approach (antisense RNAs, short hairpin RNAs (shRNAs)) to reduce the pool of translatable mRNAs
- Existing Repressor-Operator (for example TetR-TetO)
- Designing new repressors (for example Zinc Finger Proteins)
Selected System
Considering the modularity as the first criterium for the selection of the system, we considered that the Zinc Finger Protein approach would be the optimal. Indeed, the operators could be as short as 9 nucleotide long and the specificity can be easily increased by either increasing the number of fingers in the ZFP or by coupling ZFP together via Leucine Zippers. If were to use known repressor such as tetR, we would have to modify the promoter regions of the lambda-system, Pr and Prm. Although a valid approach it is dropped, due to increased workload, cost and lack of modularity
We chose the approach to use design new repressors using zinc finger domains. Furthermore, we put the binding sites for the repressors directly after start of transcription and before the ribosome binding site to prevent RNA polymerase to transcribe the gene. This would allow us to put any promotor in from of the binding site and allowing a very high degree of modularity.
If we want to use the possibility to use several 4SD connected to each order in order to count n^2, then for every new 4 state device four new repressors are needed. With zinc finger domains the construction of any binding site is possible and we will not run out of repressor.
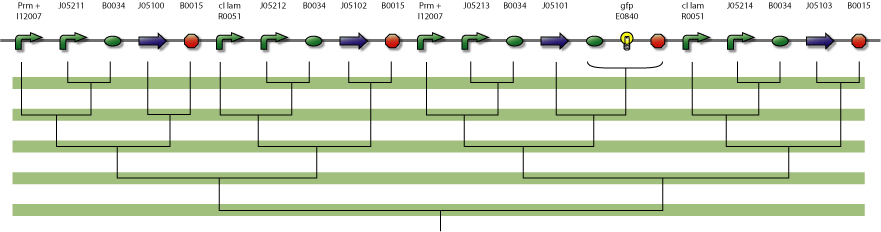
Actual Implementation
The central part of the counter is the 4SD. When input changes the next state is reached and the repressor is expressed. Every Repressor represses all other states except for the next. For example R1 represses R3 and R4, but not R2. This forces a progression of states R1 to R4 during the cycles of the input.
In the figure below is a parts-view of the device with PoPS interfaces (i/o).
Basic Functionality
The 4-State Device uses two inputs to sequentially switch through its four states. Note that this behaviour can be observed abstractly in the concept section.
To achieve such behaviour in our system, we use four interconnected gates. In the following, we call them NOR gates. Each of our NOR gates has three inputs. All of them have to be low for the output to be high.
Electrical NOR gate
A NOR gate is a very common part in electrical engineering.
pic of NOR gate
Biological NOR gate
Biologically, a NOR gate can be implemented through a promoter with high basal activity that is repressed by three effectors. If there is at least one of the three repressors, transcription is inhibited. Each NOR gates, expresses a repressor protein. (Note that such a biological NOR gate has no POPs interfaces)
biological picture of NOR gate
4-State Device circuit diagram
circuit diagram
4-State Device parts diagram
parts diagram
In the design of the Event Processing Device, input 1 and input 2 have opposite activity, meaning that either R1/R3 or R2/R4 is active. Furthermore, since R1 and R3 (respectively R2 and R4) are repressing each other, only one of the two is active. Therefore, in a stable situation, only 1 of the 4 repressor proteins is expressed.
Let us assume that R1 is being expressed. Input 2 must then be low, and therefore input 1 high. This situation is stable and remains until there is a change in the inputs. Now, if input 1 decreases, and input 2 increases, the expression of R1 will come to a halt. Since input 1 is now low, either R2 or R4 will be expressed. At this stage, R1 is still present in relatively high concentration and by repressing R4, it tips the balance in favor of R2, leading to a new stable state in which only R2 is expressed.
Note that electrical engineers call such a device a "J-K flip flop". It can also be seen as a combination of two toggle switches (Atkinson03), each being able to store one bit.
Biological Details
In the design of the repressor protein we use the following domains:
- Zinc Finger (ZF) A Zinc Finger (ZF) [http://en.wikipedia.org/wiki/Zinc_finger] is a protein domain [http://en.wikipedia.org/wiki/Protein_domain] that binds to three base pairs of double stranded DNA. A Zinc Finger Protein (ZFP) consists of one or several zinc finger domains. Many protein-DNA interaction for ZF domains and triplet of base pairs have been described, therefore making it possible to to construct artificial transcription factors by combining ZF domains in a modular fashion.
- NTD The N-terminal polymerase domain from NusA. This protein has a RNA polymerase halting properties.
- ERD
ERF repressor domain. a repressor domain in eukaryotes.
-LZ Leucine zipper. For dimerization.
If it turns out that repression has been used in all the literature we can find, then we would be better off to play safe and include them:
- Beerli PNAS 1998: they fused ZF to KRAB repressor and it has a stronger effect compare to ZF alone (see p14632 graph A)
- Beerli Nat Biotech review feb2002 : if you read the complete paragraph on gene repression (p 132), it gives strong evidence that we should fuse the ZF to a repressor (apparently at the N-Term of the ZF)
It is stated that polymerase blockade through ZF only is not very efficient. We can not be sure that the 45 amino acid long KRAB domain will work, if it doesn't exist in prokaryotes. KRAB domains have a very specific interaction with a co-repressor molecule. It might be better to find something already known to work in bacteria or skip the repressor approach altogether.
The different designs of repressors
BioBrick Part Number of ZF domains LZ domain TF domain
BBa_J05101 3 - -
BBa_J05108 3* CREBH -
BBa_J05109 3* ATF6 -
BBa_J05110 3* YAP7 -
BBa_J05111 3* cMaf -
BBa_J05112 3 - ERD
BBa_J05113 3* ATF6 KRAB
BBa_J05114 3* ATF6 NTD
BBa_J05115 6 - -
BioBrick parts
BBa_J05040 4-State Device
BBa_J05108 Repressor R1-CREBH
BBa_J05109 Repressor R3-ATF6
BBa_J05110 Repressor R2-YAP7
BBa_J05111 Repressor R4-cMaf
BBa_J05215 Regulator for R1-CREBH
BBa_J05216 Regulator for R3-ATF6
BBa_J05217 Regulator for R2-YAP7
BBa_J05218 Regulator for R4-cMaf
BBa_J05311 R1 state
BBa_J05312 R3 state
BBa_J05313 R2 state
BBa_J05314 R4 state
BBa_J05000 Zinc Finger Protein Tester
BBa_J05112 Repressor R3-e2+ERD
BBa_J05113 Repressor R3-ATF6+KRAB
BBa_J05114 Repressor R3-ATF6+NTD
BBa_J05101 Repressor R3-e2
BBa_J05115 Repressor ZF-2*e2
BBa_J05221 Tripple Binding Site for R3-ATF6
BBa_J05222 ZF-2*e2 Binding Site
BBa_J05216 Regulator for R3-ATF6
| Nickname | Length | SacI | KpnI | NheI | EcoRI | XbaI | SpeI | PstI |
|---|---|---|---|---|---|---|---|---|
| BBa_J05215+BBa_J05108 | 603 | 1 | 91 | 598 | 7, 97 | 22, 112 | 71, 578 | 85, 592 |
| BBa_J05216+BBa_J05109 | 582 | 1 | 91 | 577 | 7, 97 | 22, 112 | 71, 557 | 85, 571 |
| BBa_J05217+BBa_J05110 | 609 | 1 | 91 | 604 | 7, 97 | 22, 112 | 71, 584 | 85, 598 |
| BBa_J05218+BBa_J05111 | 600 | 1 | 91 | 595 | 7, 97 | 22, 112 | 71, 575 | 85, 589 |
| BBa_J05221+BBa_J05101 | 498 | 1 | 112 | 493 | 7, 118 | 22, 133 | 92, 473 | 106, 487 |
| BBa_J05114 | 854 | 1 | 849 | - | 7 | 22 | 829 | 843 |
| BBa_J05112 | 524 | 1 | 519 | - | 7 | 22 | 499 | 513 |
| BBa_J05222+BBa_J05115 | 676 | 1 | 87 | 671 | 7, 93 | 22, 108 | 67, 651 | 81, 665 |
The provider of the synthesized DNA used for the zinc fingerers has a lower cost limit of $500. To reduce cost per base pair for short sequences we put the short sequences together with a long sequence with restriction sites between them. the trick we use to reduce cost, by having an extra step of cloning.
binding of ZFP. describe choice of binding sites. Describe the function of RNA pol II. emphasize: modularity! Another important aspect is the overall goal of keeping the design modular - one of the most important aspects of the this contest.
We have two alternatives for placing the binding sites.
1. Binding sites in the promoter. This would prevent the polymerase from binding to the promotor. Although this might be most likely to work, we have chosen to not pursue this alternative, while we are quite intrigued by the possibility of a roadblock. The operator regions (i.e. the "roadblocks" that will prevent of the RNApolymerase to transcribe the gene) form a BioBrick that should be inserted between the promoter region and the ribosome binding site in order to keep the design modular.
2. Binding sites directly after the start of transcription and before the ribosome binding site. This alternative is attractive, since it would allow for a high degree of modularity. In theory the ZFP would act as an extra "roadblock-operator" and any promotor could be used in front of the protein.
The ZFP roadblock operator regions (from now on refered to as: operators) consists of binding motifs for two repressors. The two bindning motifs are usually spaced with 5 base pairs (gcgcg). Some data of the binding strength were available and we have chosen operators with the affinity estimated to K_d = 3-40 nM.
| Part | Binding Site Seq | est Kd [nM] | # exact matches | Hamming Dist 1 | Hamming Dist 2 | Hamming Dist 3 |
|---|---|---|---|---|---|---|
| BBa_J05100 | ggaggggac | 4 | 5 | 267 | 6196 | 65733 |
| BBa_J05102 | ggaggcggg | 30 | 17 | 799 | 12589 | 95996 |
| BBa_J05101 | gggggcgag | 3 | 15 | 634 | 10173 | 82956 |
| BBa_J05103 | ggggccgga | 45 | 44 | 848 | 11317 | 91422 |
| BBa_J05108 | gtcccctccggaggggac | N/A | 0 | 0 | 0 | 1 |
| BBa_J05109 | ctcgcccccgggggcgag | N/A | 0 | 0 | 0 | 2 |
| BBa_J05110 | cccgcctccggaggcggg | N/A | 0 | 0 | 0 | 4 |
| BBa_J05111 | tccggccccggggccgga | N/A | 0 | 0 | 0 | 6 |
| BBa_J05115 | gggggcgaggggggcgag | N/A | 0 | 0 | 0 | 3 |
Kd values of interactions. put in table above.
Degradation...?
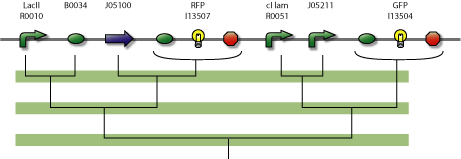
Tests: design all tests. To test whether our assumptions about using multiple zinc finger proteins (ZFP) as repressors (i.e. roadblocks) will actually work, we will build a tester/debugging device in parallel with the counter.
| Repressor | Operator | Comments |
|---|---|---|
| J05100 | J05212 | ... |
| J05100 | J05212 | ... |
4SD system
The 4SD system consists of 4 proteins with 4 operator regions. It has an interface boundary with input module (Pr and Prm). Repressor R3 is connected to a reporter to be able to count to modulo two.
assembly diagram of device.