1. Seek and Destroy Coli
From 2006.igem.org
PeterNguyen (Talk | contribs) (→Rationale) |
PeterNguyen (Talk | contribs) |
||
| Line 1: | Line 1: | ||
| - | |||
Updated 07/10/2006 | Updated 07/10/2006 | ||
| - | == | + | == Overview == |
The objective of this project is to engineer Escherichia coli which are able to actively pursue and mark or eliminate another bacterial target. This system can be divided into three components: an input element, a processing element, and a response element. The input element will consist of a quorum sensing circuit which would allow specific detection of the bacterial target. The processing element will facilitate the signaling of this input into controlled responses. A number of different response elements can be conceived, used to be used separately or in tandem: 1) integration into the chemotactic pathway of E. coli, allowing for directed mobilization towards the target, 2) reporter response at high pheromone concentrations to allow for visual identification of the target, and 3) an elimination response to produce molecules which are specifically lethal to the desired target. Such a feat will demonstrate the ability to engineer complex behavioral phenotypes (predatory-prey behavior) into bacterial systems by the integration of biological modules (quorum sensing, chemotaxis, etc.). | The objective of this project is to engineer Escherichia coli which are able to actively pursue and mark or eliminate another bacterial target. This system can be divided into three components: an input element, a processing element, and a response element. The input element will consist of a quorum sensing circuit which would allow specific detection of the bacterial target. The processing element will facilitate the signaling of this input into controlled responses. A number of different response elements can be conceived, used to be used separately or in tandem: 1) integration into the chemotactic pathway of E. coli, allowing for directed mobilization towards the target, 2) reporter response at high pheromone concentrations to allow for visual identification of the target, and 3) an elimination response to produce molecules which are specifically lethal to the desired target. Such a feat will demonstrate the ability to engineer complex behavioral phenotypes (predatory-prey behavior) into bacterial systems by the integration of biological modules (quorum sensing, chemotaxis, etc.). | ||
| Line 45: | Line 44: | ||
| - | == | + | == Proposed Experiments == |
'''Experiment 1''': Can the ComP/ComA system be used in E. coli to detect ComX? Transform E. coli with vector 1 expressing ComP and ComA, and vector 2 with a reporter gene under control of the ComA transcriptional activator. Analyze phenotype on swarming plates using B. subtilis or artificially produced ComX as bait. It is currently unknown whether this genetic system can function properly in E. coli. | '''Experiment 1''': Can the ComP/ComA system be used in E. coli to detect ComX? Transform E. coli with vector 1 expressing ComP and ComA, and vector 2 with a reporter gene under control of the ComA transcriptional activator. Analyze phenotype on swarming plates using B. subtilis or artificially produced ComX as bait. It is currently unknown whether this genetic system can function properly in E. coli. | ||
Revision as of 17:31, 10 July 2006
Updated 07/10/2006
Contents |
Overview
The objective of this project is to engineer Escherichia coli which are able to actively pursue and mark or eliminate another bacterial target. This system can be divided into three components: an input element, a processing element, and a response element. The input element will consist of a quorum sensing circuit which would allow specific detection of the bacterial target. The processing element will facilitate the signaling of this input into controlled responses. A number of different response elements can be conceived, used to be used separately or in tandem: 1) integration into the chemotactic pathway of E. coli, allowing for directed mobilization towards the target, 2) reporter response at high pheromone concentrations to allow for visual identification of the target, and 3) an elimination response to produce molecules which are specifically lethal to the desired target. Such a feat will demonstrate the ability to engineer complex behavioral phenotypes (predatory-prey behavior) into bacterial systems by the integration of biological modules (quorum sensing, chemotaxis, etc.).
Rationale
Input Element The quorum sensing system of gram negative bacteria, such as E. coli, is mediated by the production and detection of acylated homoserine lactones (AHL) which accumulate in the extracellular medium as the cellular density increases. In contrast, gram positive bacteria such as Bacillus subtilis use excreted peptide pheromones to achieve a quorum response. Due to the considerable differences between these systems, crosstalk would not be expected to occur. I propose to engineer a response element in E. coli which is able to detect the peptide pheromone of gram positive cells and produce an intracellular signal.
The ComX quorum-sensing system in gram positive bacteria consist of the genes comX, comQ, comP, and comA (Figure 1) which have been shown to regulate genetic competence. These genes cluster occur as an operon in B. subtilis and their protein products have been characterized (1).
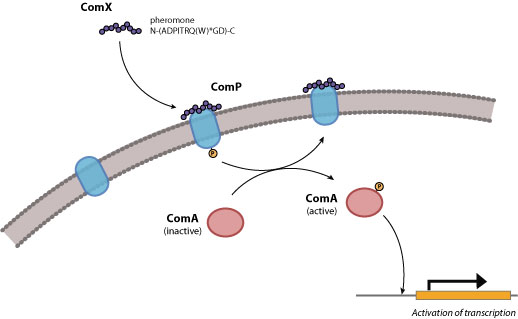 Figure 1. Quorum response in gram-postitive bacteria.
Figure 1. Quorum response in gram-postitive bacteria.
The input element will consist of the ComX pheromone, which is a 10-residue peptide secreted extracellularly. In B. subtilis, ComX production requires the presence of comQ. The exact function of ComQ is not well understood. As ComX accumulates extracellularly, detection of cell density is mediated by a ComX receptor, ComP, which is a membrane-bound histidine kinase. ComP autophosphorylates upon binding to ComX and subsequently donates this phosphate to the downstream transcriptional activator, ComA. Once activated, ComA binds to specific binding sites in the regulatory regions of genes involved in genetic competence, antibiotic production, biofilm formation, swarming motility, and membrane secretion (Comella and Grossman, 2005).
Processing Element A variety of processing elements can be integrated at the genetic level to obtain either simple gradient responses, stable biphasic switches (toggle switch) or integration with another input (AND gates). Many of these modules have been constructed and tested in various iGEM projects. The usage of these various inputs to modulate the system response will be initially simulated using MATLAB Simulink and fine-tuned during empirical testing of the system.
Response Element A. Quorumtaxis Chemotaxis in bacteria has been extensively studied as a model system for receptor signaling and intracellular signal transduction. To actively seek energy sources or optimal physicochemical environments, bacteria such as E. coli employ a system of alternating tumbling and swimming behaviors. Detection of an extracellular chemical signal produces a swimming phenotype (CCW flagellar rotation); a decrease in the signal induces a tumbling response (CW). The bacterium thus traces out a path similar to a ‘random walk,’ which produces movement in the direction of increasing attractant. Feedback control by methylation of the receptor proteins restores sensitivity to the environmental input by modulating phosphorylation activity. This allows the cell to detect attractant gradients in space as well as time (2). The chemotaxis system has also been shown to be essential for swarming motility in E. coli (3). By integrating quorum-sensing and chemotactic pathways, it is possible to engineer a ‘quorumtaxis’ system, whereby a bacterium can sense the quorum pheromone of another species and move in that direction. To date, there have been no published results of engineered chemotaxis systems. Construction of a functioning quorumtaxis system would be a significant innovation that demonstrates the power of developing a novel complex phenotype from pre-existing bacterial components. This can be done by integration of the ComX receiver system composed of ComP/ComA with the chemotaxis system of E.coli (figure 3). This engineered E. coli will begin to migrate towards gram positive cells (e.g., B. subtilis) due to the presence of extracellular ComX pheromone produced by these cells. Also, by adding the AHL quorum-sensing system to the E. coli cells and integrating the AHL and ComX detection systems together, it would be possible to produce an output which is dependent on both the proximity to the prey cells and the density of the predator strain.
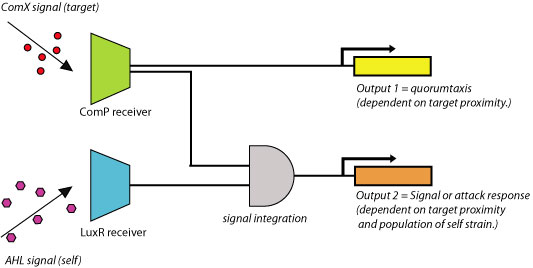 Figure 2. Example of simple quorumtaxis circuit.
Figure 2. Example of simple quorumtaxis circuit.
The difficult aspect of this proposed project would be to construct and optimize the quorumtaxis pathway. Flagellar response to the ComP receiver can be engineered in three ways:
1. Construction of a ComP-CheA chimeric mutant. In this scheme, a library of ComP-CheA chimeras would be produced and tested for the ability to elicit straight swimming in E. coli in the presence of extracellular ComX. On a molecular scale, the desired chimeric protein would be able to bind ComX and possess an allosterically controlled histidine kinase activity. The appropriate fusion protein should consist of 1) the sensor domain of ComP, 2) a transmembrane region, and 3) the cytoplasmic signaling domain of an E. coli chemoreceptor, such as Tsr. Figure 4 shows the domains of This strategy is similar to that employed by the Voight laboratory, to produce a phytochrome-EnvZ histidine kinase chimeric protein in which red light allosterically inhibits autophosphorylation and shuts off gene expression. The advantage of this approach is that it retains all the functional elements of the bacterial chemotaxis system; the disadvantage is that a major part of the project will involve protein engineering and screening.
2. Expression of a consitutively active CheY mutant under control of ComA activation. One can envision an alternate strategy in which a chemotaxis-inducing protein is placed under control of the ComA activator. This would bypass the inherent difficulty in developing a chemotaxis phosphorylation cascade under control of a quorum receptor. Previous studies on the CheY protein, which is the direct effector of flagellar motor proteins, have resulted in the identification of several CheY mutants with interesting properties. The mutant CheYD57A produces cells which are constitutive swimmers, and the mutant CheYD13K produces cells which are constitutive tumblers. Both of these CheY mutants induce these phenotypes without phosphate-mediated activation. Thus, binding of the ComX pheromone by ComP would activate ComA, which would drive the expression of CheYD57A while repressing CheYD13K, resulting in straight swimming. The advantages to this approach would be the ease of implementation – regulated single proteins give rise to a desired phenotype. However, the caveats include a loss in the sensitivity of the chemotaxis system, due to the circumvention of elements involved in motor response adaptation speed (CheZ) and gradient response (CheB and CheR). In addition, engineering of a CheY-controlled motor response would require the use of an E. coli strain in which the che genes have been deleted (PS2002).
3. Expression of Tsr fragments under control of ComA activation. Another alternative would be to express an upstream chemotaxis signaling protein under control of ComA. The serine chemoreceptor Tsr, like most methyl-accepting chemotaxis proteins (MCPs), are transmembrane proteins roughly 550 amino acids in length. They contain a periplasmic ligand-binding domain, a transmembrane domain, and a cytoplasmic signaling domain. Tsr is one of the major chemoreceptors in E. coli and is highly expressed (4). Ames and Parkinson have demonstrated that soluble fragments of the Tsr cytoplasmic signaling domain (residues 290-470) expressed in E. coli Tsr deletion strains (RP5700) are able to alter flagellar rotation (5). Specifically, wildtype Tsr fragments are known to produce a CW signal (tumbling), whereas the same fragment with a single amino-acid mutation A213V shifts the flagellar rotation to CCW (straight swimming). Thus, by placing the expression of Tsr-CCW under the control of the ComA transcriptional activator, it may be possible to integrate the detection of ComX pheromone with smooth forward swimming.
Signaling/Kill response
The engineering of quorumtaxis in E. coli would be the primary task at hand. Construction of downstream functions would be relatively straightforward. For example, if the bacterium is able to detect and swarm towards the gram-positive target cells, we could then design the E. coli to fluoresce (signaling) and/or express antimicrobial peptides (elimination). A number of defensins, such as those produced by certain insects, exhibit bactericidal activity against gram-positive bacteria. Another potential module for a kill response could be the daptomycin biosynthesis gene cluster, which produces a 13 amino acid cyclic lipopeptide with potent activity against gram-positive bacteria.
Proposed Experiments
Experiment 1: Can the ComP/ComA system be used in E. coli to detect ComX? Transform E. coli with vector 1 expressing ComP and ComA, and vector 2 with a reporter gene under control of the ComA transcriptional activator. Analyze phenotype on swarming plates using B. subtilis or artificially produced ComX as bait. It is currently unknown whether this genetic system can function properly in E. coli.
Experiment 2a: Can functional ComP/Tsr hybrids be engineered? Using PCR methods, a number of chimeras will be engineered, fusing the ComX-sensing domain of ComP with the cytoplasmic domain of the Tsr receptor. Successful mutants will be able to produce a quorumtactic response in the presence of ComX. Screening will be performed in E. coli Tsr deletion mutants.
Experiment 2b: Does expression of Tsr or CheY-mutant fragments allow sufficient control of the E. coli chemotactic pathway? Expression of Tsr or CheY fragments known to alter the flagellar rotation of E. coli will be placed under control of the ComP-ComA system. Thus, in the presence of ComX, the appropriate protein will be expressed to control quorumtaxis.
Proposal Impact
Novelty: 1. This will be the first demonstration of a novel phenotype: quorumtaxis, the ability of a bacterium to modify its movement towards a quorum-sensing pheromone. 2. The novel integration of quorum-sensing and chemotaxis would be a significant advance in the field of synthetic biology. 3. Progress on this project would improve the tools available for engineering chemotactic responses to unnatural ligands, which is currently non-existent.
New parts to be added to iGEM registry: 1. ComX quorum sensing system. This part would consist of a 2-gene construct (ComQ, ComX) for the sender device and a 2-gene construct for the receiver (ComP, ComA). Currently, the iGEM registry only contains AHL-based systems of the LuxR receiver family. The addition of a completely different quorum system would greatly expand the tools available for cell-to-cell communication. 2. ComP-Tsr chimera. This protein would link chemotactic pathways with the detection of a ligand which is not normally detected in E. coli. 3. Tsr / CheY mutants. Although these protein fragments already exist, placing them in the iGEM registry would allow users to place chemotaxis under transcriptional control.