Background
From 2006.igem.org
| Line 10: | Line 10: | ||
<h3><font face="Broadway, Verdana">Introduction</font></h3> | <h3><font face="Broadway, Verdana">Introduction</font></h3> | ||
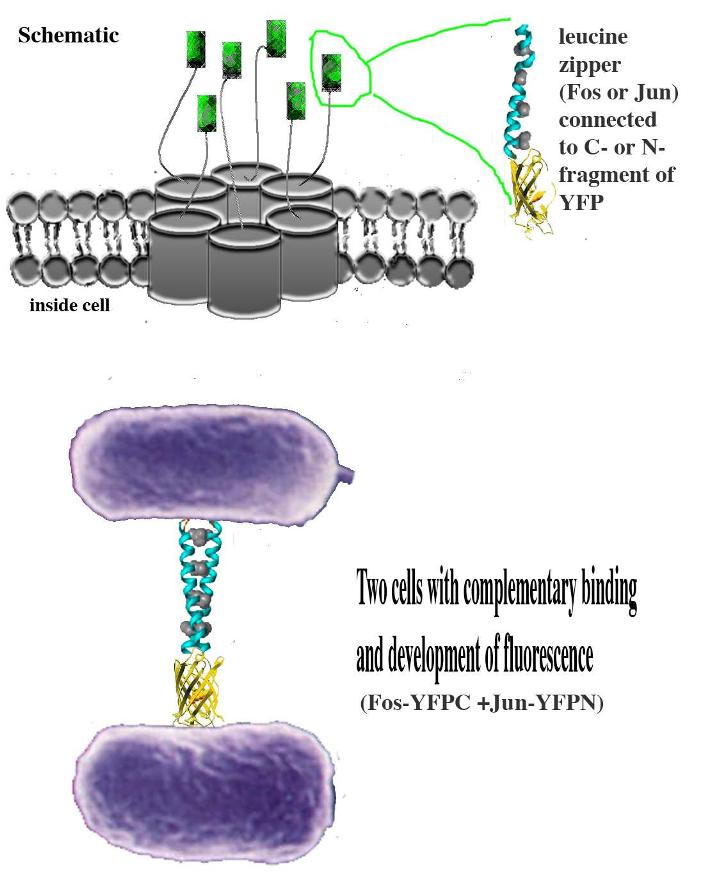
| - | The idea behind the project is fluorescence complementation, which involves the joining of two leucine zipper proteins, Fos and Jun, each fused to a half terminus of YFP. Originally, the Fos and Jun proteins were fused to a beta gene coding for a membrane protein. The project involved performing a PCR reaction to produce two inserts, the N-terminus and the C-terminus of | + | The idea behind the project is fluorescence complementation, which involves the joining of two leucine zipper proteins, Fos and Jun, each fused to a half terminus of YFP. Originally, the Fos and Jun proteins were fused to a beta gene coding for a membrane protein. The project involved performing a PCR reaction to produce two inserts, the N-terminus and the C-terminus of YFP, and then ligating these inserts into 2 vectors, containing Jun-beta and the Fos-beta respectively. The two fusion proteins (Fos-beta-YFPC and Jun-beta-YFPN) were expressed in the cell membrane of two populations of <i>E. coli</i>. We then allowed these two cell types to combine, resulting—ideally—in the complementary binding of the Jun and Fos proteins when the cells are in close contact. Consequently, the two half YFP fragments bind to form full YFP, and the cells will fluoresce. |
Latest revision as of 17:49, 29 October 2006

Fluorescence complementationBack to McGill University Main Page
IntroductionThe idea behind the project is fluorescence complementation, which involves the joining of two leucine zipper proteins, Fos and Jun, each fused to a half terminus of YFP. Originally, the Fos and Jun proteins were fused to a beta gene coding for a membrane protein. The project involved performing a PCR reaction to produce two inserts, the N-terminus and the C-terminus of YFP, and then ligating these inserts into 2 vectors, containing Jun-beta and the Fos-beta respectively. The two fusion proteins (Fos-beta-YFPC and Jun-beta-YFPN) were expressed in the cell membrane of two populations of E. coli. We then allowed these two cell types to combine, resulting—ideally—in the complementary binding of the Jun and Fos proteins when the cells are in close contact. Consequently, the two half YFP fragments bind to form full YFP, and the cells will fluoresce.
Back to McGill University Main Page |