X-Y chemotaxis
From 2006.igem.org
| Line 1: | Line 1: | ||
[[NCBS_Bangalore_2006#The_Living_Networks_Wiki|IGEM Bangalore HOME]] | [[NCBS_Bangalore_2006#The_Living_Networks_Wiki|IGEM Bangalore HOME]] | ||
| - | + | ==Group I== | |
| + | ===Aim=== | ||
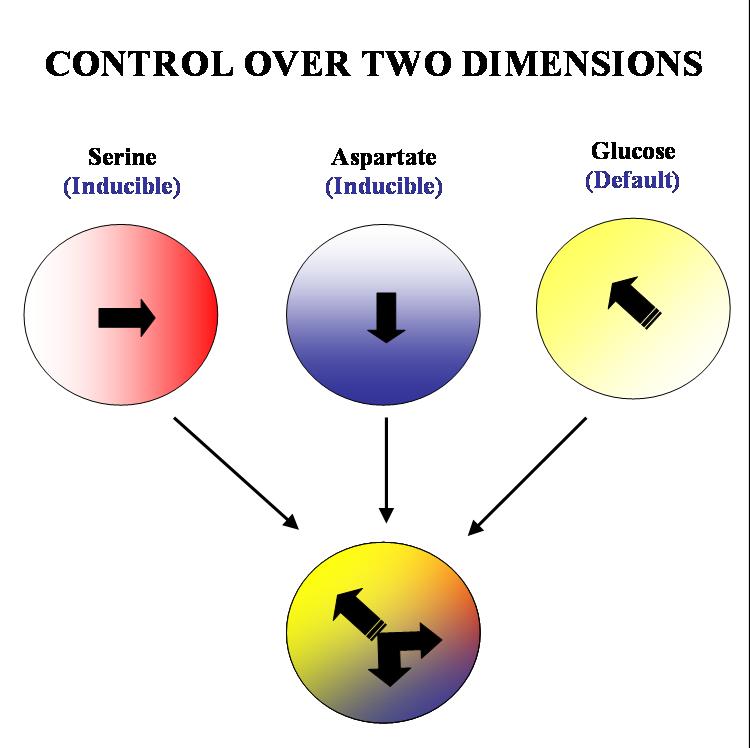
| - | + | Our ultimate aim is to get complete two dimensional control over the movement of colonies of ''E. coli.'' on a solid substrate such as an agar plate. We plan to acheive this by 'hacking' into the existing chemotactic machinery in the ''E. coli'' cell. Specifically, if we are able to independently and efficiently alter the sensitivity of our bugs to stable orthogonal gradients of different amino acids, it will be possible to implement such control. | |
| - | + | ||
| + | ===Approach=== | ||
| + | |||
| + | The specific geometry we have in mind is the following: there will be two stable gradients of serine and aspartate, orthogonal to each other. By inducing the expression of Tar and Tsr chemotaxis receptors (Tar for aspartate and Tsr for serine) in a null background (UU1250 strain) we hope to independently alter the response of the bug to these two gradients. There will also be a third gradient of glucose angled at 135 degrees to each of the amino acid gradients. Glucose is a PTS (phosphotransferase system) sugar and doesn't require Tar/Tsr receptor expression for its detection. Hence the bug will be constitutively responsive to glucose and this will serve as the default negative direction. | ||
[[Image:3gradients.jpg|thumb|left|Gradient assay]] | [[Image:3gradients.jpg|thumb|left|Gradient assay]] | ||
| Line 19: | Line 23: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | <br> | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ===Constructs=== | ||
| + | |||
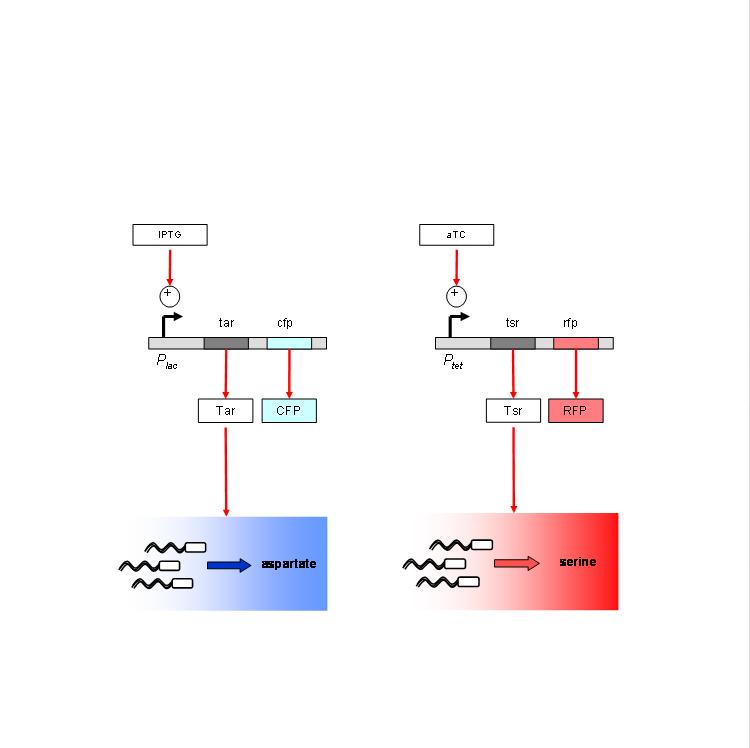
| + | We are using the following constructs for induction of expression of Tar and Tsr. Tar expression is controlled by the lactose promoter, while Tsr expression is controlled by the Tet promoter. CFP and RFP provide readouts of relative transcript levels. We specifically chose these two reporters because of their spectral spacing in order to minimize bleed-through during fluorescence imaging. | ||
| + | |||
| + | [[Image:constructs_chemotaxis.jpg|thumb|left|Chemotaxis contructs]] | ||
| + | The chemotaxis null strain, UU1250, first had to be transduced with chromosomal copies of the genes coding for repressor molecules for each of the promoters - lacI for the lac promoter, and TetR for the Tet promoter. This was done using P1 phage transduction with the host strain being K12Zi. The selection marker was Spectinomycin. We selected 4 strains of the (lacI-TetR) transduced UU1250, named UU1250zi 4 through 7, and went ahead to test independent induction. | ||
| + | |||
| + | <br> <br> <br> <br> <br> <br> <br> <br> <br> | ||
| + | |||
| + | ===Induction=== | ||
| + | |||
| + | |||
| + | |||
| + | |||
Attempts to establish such a gradient(s) using technigues standardised earlier include the slant plate. A KMnO4 gradient set-up is shown here: | Attempts to establish such a gradient(s) using technigues standardised earlier include the slant plate. A KMnO4 gradient set-up is shown here: | ||
| Line 35: | Line 57: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 10:18, 29 October 2006
Contents |
Group I
Aim
Our ultimate aim is to get complete two dimensional control over the movement of colonies of E. coli. on a solid substrate such as an agar plate. We plan to acheive this by 'hacking' into the existing chemotactic machinery in the E. coli cell. Specifically, if we are able to independently and efficiently alter the sensitivity of our bugs to stable orthogonal gradients of different amino acids, it will be possible to implement such control.
Approach
The specific geometry we have in mind is the following: there will be two stable gradients of serine and aspartate, orthogonal to each other. By inducing the expression of Tar and Tsr chemotaxis receptors (Tar for aspartate and Tsr for serine) in a null background (UU1250 strain) we hope to independently alter the response of the bug to these two gradients. There will also be a third gradient of glucose angled at 135 degrees to each of the amino acid gradients. Glucose is a PTS (phosphotransferase system) sugar and doesn't require Tar/Tsr receptor expression for its detection. Hence the bug will be constitutively responsive to glucose and this will serve as the default negative direction.
Constructs
We are using the following constructs for induction of expression of Tar and Tsr. Tar expression is controlled by the lactose promoter, while Tsr expression is controlled by the Tet promoter. CFP and RFP provide readouts of relative transcript levels. We specifically chose these two reporters because of their spectral spacing in order to minimize bleed-through during fluorescence imaging.
The chemotaxis null strain, UU1250, first had to be transduced with chromosomal copies of the genes coding for repressor molecules for each of the promoters - lacI for the lac promoter, and TetR for the Tet promoter. This was done using P1 phage transduction with the host strain being K12Zi. The selection marker was Spectinomycin. We selected 4 strains of the (lacI-TetR) transduced UU1250, named UU1250zi 4 through 7, and went ahead to test independent induction.
Induction
Attempts to establish such a gradient(s) using technigues standardised earlier include the slant plate. A KMnO4 gradient set-up is shown here: