Lab Work
From 2006.igem.org
(→13th July 2006) |
(→13th July 2006) |
||
| Line 7: | Line 7: | ||
{| border="1" | {| border="1" | ||
| - | |||
!width="100"| | !width="100"| | ||
!width="100"| | !width="100"| | ||
| - | !width=" | + | !width="100"| |
| + | !width="100"| | ||
| + | |- | ||
| + | | Parameter: || 50% culture || 25% culture || 12.5% culture | ||
| + | |- | ||
| + | | Ampicillin: || 50 microlitres || 50 microlitres || 50 microlitres | ||
| + | |- | ||
| + | | IPTG: || 50 microlitres || 50 microlitres || 50 microlitres | ||
| + | |- | ||
| + | | Culture: || 25 ml || 12.5 ml || 6.25 ml | ||
|- | |- | ||
| - | | | + | | Sterile water: || 25 ml || 37.5 ml || 43.75 ml |
|} | |} | ||
Revision as of 09:07, 14 July 2006
13th July 2006
We performed ligations with the DNA purified from the colonies transformed on the 6th, 7K (promoter) to 30 (RBS) and 9E (lacZ) to 1I (terminator). The recombinant plasmids were transformed (hopefully) into competent cells and plated on medium containing Xgal and IPTG, with a pBluescript colony also plated as a control. We also suspended more original colonies in liquid culture as a backup.
The primers ordered for a better lacZ gene, and the arsR and ars promoter arrived and we did PCR with cells of two different E. coli strains.
In order to determine the ideal conditions to achieve a pH response, three different growth mediums were established:
| Parameter: | 50% culture | 25% culture | 12.5% culture |
| Ampicillin: | 50 microlitres | 50 microlitres | 50 microlitres |
| IPTG: | 50 microlitres | 50 microlitres | 50 microlitres |
| Culture: | 25 ml | 12.5 ml | 6.25 ml |
| Sterile water: | 25 ml | 37.5 ml | 43.75 ml |
12th July 2006
The pH response over time was again measured but this time, we used liquid cultures which were already saturated (i.e. in the stationary phase). The results will be put up soon.
11th July 2006
Today we tested the timed pH response in 2 cell cultures: 1 with the LacZ gene, which was activated by IPTG, and one that had this gene absent.
We cut the isolated plasmid DNA with restriction enzymes to remove the inserts from the promoter and lacZ part, and open the vectors for the RBS and terminator. We ran gels, and these showed that the restriction had succeeded, but had not yielded much DNA. The correct bands were cut out of the gel to purify the inserts and vectors, ready for ligation.
10th July 2006
The colonies transformed on the 6th made it this time, and we isolated the plasmid DNA from three individual colonies for each biobrick. We also transformed some E. coli with
| 23E | pSB1A3 | Plasmid | Plate 1 | AmpR |
to serve as an empty plasmid for the new LacZ and arsenic promoter/repressor parts which we will create.
7th July 2006
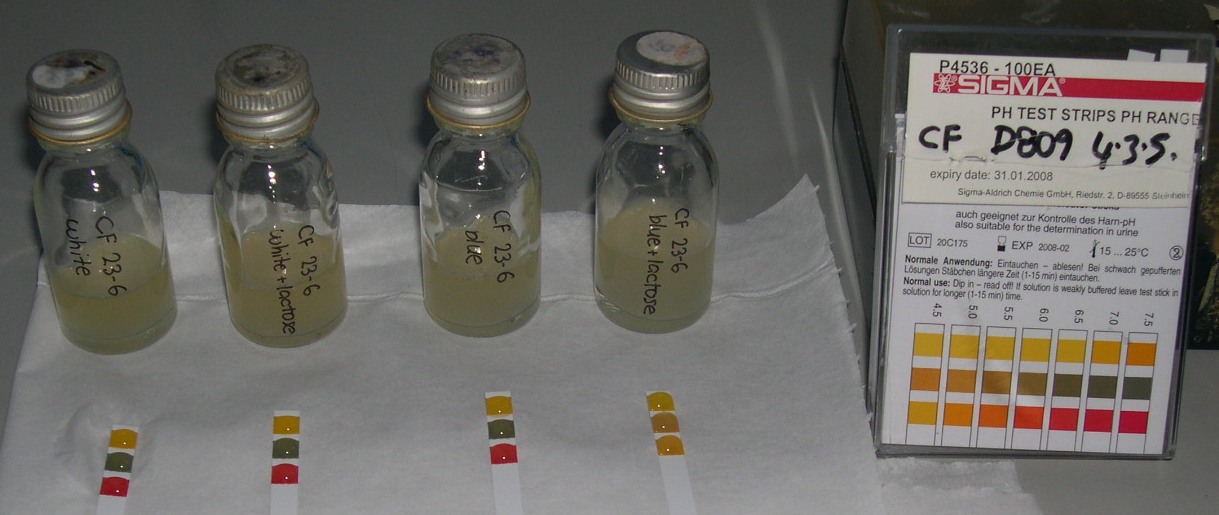
When bacteria with the lacZ gene inserted are present in a medium containing lactose, the pH does drop significantly.
6th July 2006
Unfortunately the colonies we plated on the 4th did not survive due to a problem with the competent cells we used, so today we repeated transforming and plating colonies containing the following parts:
| 9E | BBa_E0033 | LacZ alpha | Plate 2 | KanR |
| 1I | BBa_B0015 | Terminator | Plate 1 | AmpR |
| 7K | BBa_R0010 | IPTG responsive promoter | Plate 1 | AmpR |
| 3O | BBa_B0034 | RBS | Plate 1 | AmpR |
4th July 2006
We plated colonies containing plasmids with the following parts:
| 9E | BBa_E0033 | LacZ alpha | Plate 2 | KanR |
| 3P | BBa_0010 | Terminator | Plate 2 | AmpR |
| 7K | BBa_R0010 | IPTG responsive promoter | Plate 1 | AmpR |
[http://2006.igem.org/Standard_Protocols Standard Protocols]
[http://2006.igem.org/University_of_Edinburgh_2006 Main page]