Tokyo Alliance: Conclusion
From 2006.igem.org
| Line 64: | Line 64: | ||
In our assay, we measured fluorescence resonance of GFP of E.coli cultured in liquid medium with or without chemicals. | In our assay, we measured fluorescence resonance of GFP of E.coli cultured in liquid medium with or without chemicals. | ||
| - | [[Image:tokyo_data_60.jpg|400px]][[Image:tokyo_data_61.jpg|400px]] | + | [[Image:tokyo_data_60.jpg|400px]] |
| + | |||
| + | We show GFP expression regulated by yes gate containing lacI binding sites. | ||
| + | |||
| + | In this graph, X-axis is induction time. Y-axis is GFP fluorescence. | ||
| + | |||
| + | This white arrow indicates GFP expression of E.coli cultured without IPTG. By addition of IPTG, we indicate it by red arrow, GFP fluorescence increased 1.5times. | ||
| + | |||
| + | |||
| + | [[Image:tokyo_data_61.jpg|400px]] | ||
| + | |||
| + | This is the construct of FadR. By addition of oleate, GFP expression increased 1.5 times. | ||
| + | |||
| + | |||
| + | [[Image:tokyo_data_62.jpg|400px]] | ||
| + | |||
| + | This is the construct of AraC. | ||
| + | By addition of arabinose, GFP expression increased 3 times. | ||
| + | |||
| + | |||
| + | [[Image:tokyo_data_63.jpg|400px]] | ||
| + | |||
| + | This is the construct of EmrR. | ||
| + | By addition of salicilate, GFP expression increased 1.5times. | ||
| - | |||
[[Image:tokyo_data_64.jpg|400px]] | [[Image:tokyo_data_64.jpg|400px]] | ||
| + | |||
| + | This is the construction of LuxR. | ||
| + | By addition of AHL, GFPexpression increased 6times. | ||
==Conclusion== | ==Conclusion== | ||
Revision as of 21:13, 29 October 2006
Contents |
Results
We searched several number of, actually at least 7, regulators inducible by different chemicals. This list is the candidates of such a regulator.
Using these regulatory sequences, we constructed these iGEM parts. There are 22 different logic gates actually made. Some of them are ANDgates.
We constructed YES gates and AND gates shown above accorinding to our Procedure.
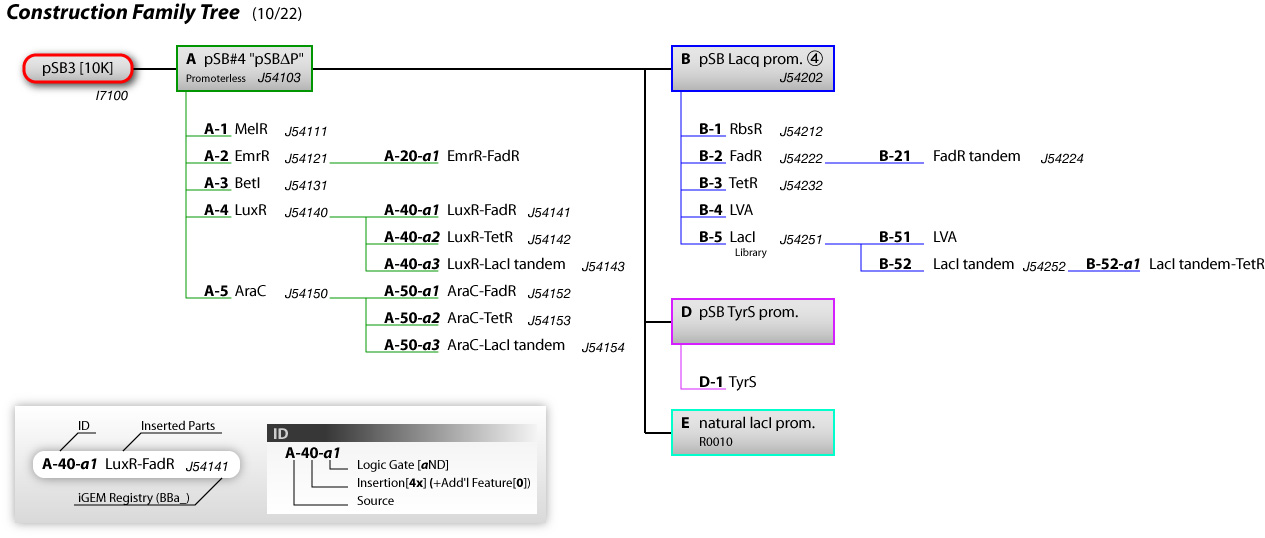
Construction Index
- A pSB#4 "pSBΔP" [http://partsregistry.org/Part:BBa_J54111 J54103]
- A-1 MelR [http://partsregistry.org/Part:BBa_J54111 J54111]
- A-2 EmrR [http://partsregistry.org/Part:BBa_J54121 J54121]
- A-20-a1 EmrR-FadR
- A-3 BetI [http://partsregistry.org/Part:BBa_J54131 J54131]
- A-4 LuxR [http://partsregistry.org/Part:BBa_J54140 J54140]
- A-40-a1 LuxR-FadR [http://partsregistry.org/Part:BBa_J54141 J54141]
- A-40-a2 luxR-TetR [http://partsregistry.org/Part:BBa_J54142 J54142]
- A-40-a3 LuxR-LacI tandem [http://partsregistry.org/Part:BBa_J54143 J54143]
- A-5 AraC [http://partsregistry.org/Part:BBa_J54150 J54150]
- A-50-a1 AraC-FadR [http://partsregistry.org/Part:BBa_J54152 J54152]
- A-50-a2 AraC-TetR [http://partsregistry.org/Part:BBa_J54153 J54153]
- A-50-a3 AraC-LacI tandem [http://partsregistry.org/Part:BBa_J54154 J54154]
- B pSB Lacq prom. #4 [http://partsregistry.org/Part:BBa_J54202 J54202]
- B-1 RbsR [http://partsregistry.org/Part:BBa_J54212 J54212]
- B-2 FadR [http://partsregistry.org/Part:BBa_J54222 J54222]
- B-21 FadR tandem
- B-3 TetR [http://partsregistry.org/Part:BBa_J54232 J54232]
- B-4 LVA
- B-5 LacI (Library) [http://partsregistry.org/Part:BBa_J54251 J54251]
- B-51 LVA
- B-52 LacI tandem [http://partsregistry.org/Part:BBa_J54252 J54252]
- B-52-a1 LacI tandem-TetR
- C pSB LacI prom. #1
- D pSB TyrS prom.
- D-1 TyrS
- E natural LacI prom.
[http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2006partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2006&group=Tokyo Tokyo Alliance iGEM registry list]
Data
In our assay, we measured fluorescence resonance of GFP of E.coli cultured in liquid medium with or without chemicals.
We show GFP expression regulated by yes gate containing lacI binding sites.
In this graph, X-axis is induction time. Y-axis is GFP fluorescence.
This white arrow indicates GFP expression of E.coli cultured without IPTG. By addition of IPTG, we indicate it by red arrow, GFP fluorescence increased 1.5times.
This is the construct of FadR. By addition of oleate, GFP expression increased 1.5 times.
This is the construct of AraC. By addition of arabinose, GFP expression increased 3 times.
This is the construct of EmrR. By addition of salicilate, GFP expression increased 1.5times.
This is the construction of LuxR. By addition of AHL, GFPexpression increased 6times.
Conclusion
- Conclusion
We can say we expanded the number of regulator genes we can use to build logic gates and through this project we made simple constructing method.
- Future Work
It must be possible to construct functioning AND gates with our systematic construction strategy. And with same method, it is possible to make ANDAND gates, also. In order to make Noughts and Crosses game, we need 11 AND gates and 10 ANDAND gates.
Not tested yet, we already have constructed 7AND gates. It may not be so hard to complete the set of logic gates and combine them into Noughts and Crosses.
We recently change transition diagram and found that the number of inputs can be reduced into just 6. So the implementation of SYANAC will be little bit easier.