Oscillator based
From 2006.igem.org
Back to the ETH Zurich main page.
Contents |
Scope
The general focus of our group is on ideas related to counters and oscillators.
Currently, our goal is to create a counter for concentration peaks (e.g. of a protein experiencing oscillatory behaviour) that is robust to variations in phase length, peak width and height, while composed of a minimal number of proteins and regulatory pathways.
Ideally, this module should be combined with, on one hand, a cell cycle dependent protein to make a generation counter, and, on the other hand, with the implementation of a synthetic oscillator developed by previous initiatives. If both integration fail, a hand-generated inducer oscillation will hopefully work as a backup and provide entertainment to our audience.
Motto
ASCII is an art form!
Organisation
People
People currently working on these Ideas:
Please feel free to add your name.
Meetings
Friday, 5th August, 09:00 @ Christophe's bureau (CAB F61.2) Summary of the meeting Monday, 8th August, 15:00 @ Christophe's bureau (CAB F61.2) Tuesday, 9th August, 09:00 @ Christophe's bureau (CAB F61.2)Summary of the meeting Wednesday, 10th August, 09:15 @ Christophe's bureau (CAB F61.2) Thurdsay, 11th August, 12:00 @ Mensa
Design
Below, we divide the concept into modules, to allow independence to the greatest extent in the design, implement and test phase of each of them. Note that all three forms of oscillators might not lead to the fluctuation of the same protein, and thus an adaptor or interface will possibly be needed.
+---------------+
| OSCILLATOR |
| ============ |
+---------------+
| |
| cell division | +-------------------+ +-------------------+
| | | | | |
+---------------+ | DIVISOR/COUNTER | | |
| oscillator |----> | =============== |----> | reporter gene |
| from previous | | | | |
| work | | | | |
+---------------+ +-------------------+ +-------------------+
| triggering |
| by hand |
| |
+---------------+
OSCILLATOR
Cell division
Uwe Sauer suggests to look into ftsZ as a protein that is expressed only once per cycle. We could try to get their promoter and use it to build a peak as input for the counter.
Uwe: FtsZ might not be the best choice. Also pivotal for cell cycle, I think a substantial fraction is present all the time, it simply assembles around the cell middle to initiate division. What you need, is actually a protein whose presence is primarily regulated at the genetic level. Perhaps FtsZ works, but I am not so sure. I would recommend to go through the literature and see whether or no there is another Fts gene (or others) that are only transiently present during division. There are a lot more proteins involved in the process and all you need is one that is transiently expressed. Reviews are a good starting point, but also hve a look at chapter 101 of the E. coli book.
Christophe: I just found a disturbing article from 1979 that is entitled "Individual Proteins are Synthesized Continuously throughout the E. Coli Cell Cycle" [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=218185], but then again, that was in 1979, a time where the sole lab instruments were handcrafted petri dishes and self-grinded magnifying lenses. Later, I found yet another disturbing article ([http://www.cnb.uam.es/~mvicente/Rueda(185)3344.pdf]) that clearly shows that the concentration of FtsZ and a few others remain constant throughout the cell cycle in E. Coli. There are many instances of oscillating proteins in other organisms, in particular caulobacter crescentus or yeasts, but so far i am out of luck in E. Coli. Yet more discouraging results! Arends and Weiss [http://jb.asm.org/cgi/content/full/186/3/880] claim in their paper that "Our results also imply that no E. coli genes are expressed in a division cycle-dependent manner"...
Alexander: Perhaps DnaA could be used as a target for creating a generation counter. DnaA binds to OriC and initiates DNA replication and later recruiting several protins (DnaB, DnaC, DnaG, ect.). The initiation of chromosomal replication in E. coli is dependent on availability of DnaA. If the concentration will fluctutate with the cellcycle then DnaA can be used as a signal to count generation. Since DnaA binds to DNA it would can be used as a operator. The idea would be to use a DnaA binding sequence as an operator in a reporter gene.
Recommended Readings
- Review on Bacterial Cell Division [http://arjournals.annualreviews.org/doi/pdf/10.1146/annurev.genet.33.1.423;jsessionid=jG4OUnl1knY7]
- Review on Microanalysis of gene expression during cell cycle [http://www.cellandchromosome.com/content/2/1/1]
- Report on Oscillating regulators, but only in C. Crecentus... [http://www.sciencemag.org/cgi/content/short/1095191v1]
- Review on Oscillating regulators with relevant modelling info, but only about S. Pombe [http://www.nature.com/nrm/journal/v2/n12/abs/nrm1201-908a_fs.html]
Oscillator
Attenuated Oscillator from Cell Article
Bad news! I read the paper about oscillatory behavior in E coli (atkinson03) and if I did get it right then they didn't completely succed to develop a stable oscillator. Their design enabled them to achieve 4 different behaviors of the circuit by changing the promoters, repressors, copy numbers and cooperativity. Due to theire simulations the circuit should have been able to act as a toggle switch, a damped oscillator and a stable oscillator (with different frequencies and peak concentrations). They succeded in establishing reproducibly a damped oscillation. But by shifting the system parameters into the region of stable oscillation the damping didn't disappear. Pushing it further could possibly help but I don't know if it's worth it and if we have enough time for this.
Furthermore they didn't include all the genes into peptides but a part also in the DNA of the E coli itself. I don't know if this would be a problem but I wanted to mention it anyway.
Repressilator
The repressilator is working but as we know it has its drawbacks. On one hand it isn't oscillation isn't really very regullary and on the other hand it's amplitude is increasing with time. With this oscillator you could perhaps count to 2 or 3. Because if the minimum amplitude is to high thecounter wouldn't recognice the difference anymore. But instead of using the simple hand triggering this could be a alternative to test the counter.
Another Project from the Registry of Standard Parts
Triggering by Hand
Let's brainstorm about stuff that could be used to generate an oscillation by hand.
- IPTG (deactivates the LacI repressor)? What about degradation time?
- Heat Shock Protein? Think about it, the second time we cook the bacteria, it reacts differently :-)
- AHL quorum sensing? But then, can we order AHL? at which price?
- ...
Uwe: I like this back-up idea very much. likely you will have to use it..... Chemical variations are always tricky because the compounds tend to linger around and you'll typicaly end up with a single on-off cycle. IPTG and AHL (but also pH etc) fall into this category unless you add degrading enzymes (..which then effect the next cycle in some way). Physical parameters offer the benefit that they can easily be modified from the outside without a 'memory' effect. I think Temp. is a great choice!
Don't worry about cooking. Heat shock is elecited by a relative raise in T, e. g. going from 30 to 37 (or perhaps 40°C). The cells will be fine and the heatshock protein-based reporter is under your external control. You can even set an automated T cycle in a PCR (or similar) machine, which will help you a lot by finging the right set of parameters for optimal performance. Also it looks cooler than working with your hands on vials..
Christophe: I looked into sigma 32 heat-shock promoters. That could work, but we still need to find some people who have experience with that, as it is likely that there will be a lot of small details required to have a proper response. Further promoters to evaluate/test: ibp, cspA,
Recommended Readings
- Introduction to Heat-Shock Response in Bacteria [http://www.tau.ac.il/lifesci/departments/biotech/members/segalg/GS-R2.pdf]
- Consensus sequence for E. Coli Heat Shock gene promoters [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3887408]
DIVISOR/COUNTER
This module will get an input concentration from the interface module and will count, due to the changing input, to 4. Also a reporter (for example different colors) will be produced which could serve as an indicater for the state the circuit is currently occupiing.
Idea and Implementation
During our discussion about the divisor implementation we noticed that the previous one wasn't quite stable. Talking about toggle switches we suddenly had the idea of combining the base structur of the toggle switch we learned to know during the crash course with the allready existing design. The following drawings show this new design which is basically a combination of two such toggle switches.
All substances which are involved should be produced and decomposed quickly
S = concentration signal from the interface
R1 is produced by gene (1) and represses gene (0) and (3)
R2 is produced by gene (2) and represses gene (0) and (1)
R3 is produced by gene (3) and represses gene (1) and (2)
R0 is produced by gene (0) and represses gene (2) and (3)
---S---
R1 / \ R3 R3 R0 R1 R2
\ | | / | | | |
\/|____ | | ____|\/ /|___ | | | | ____|\
___/ (1) |_____v__ __v_____| (3) \___ ___/ (2) |_=_=____ ____=_=_|(0=4) \___
\ ____| = = = = |____ / \ ____| = = |____ /
\| | | | | |/ /\| | | |/\
| | | | / | | \
R2 R3 R0 R1 R2 \ / R0
---S---
The following time lines should be the result of this gene circuit:
S:
----- ----- ----- ----- Let's assume that the counter is in state 0
| | | | | | | | (expressing R0). As soon as the concentration
| | | | | | | | of S goes up (0) and (2) are repressed
--- ----- ----- ----- ---- (no further expressing of R0). Now R1 can be
R1: expressed while (3) is still repressed
---- ---- by R0. The production of R1 ensures that (3)
/ \ / \ won'tbe active when R0 is totally decomposed.
/ \ / \ -> We are in state (1).
--- ---------------- --------------- When the S goes down again R1 can't be
R2: expressed anymore. Now R2 can be
---- ---- expressed while (0) is still repressed by R1.
/ \ / \ The production of R2 ensures that (0) won't
/ \ / \ be active when R1 is totally decomposed.
--------- ---------------- --------- -> We are in state (2)
R3: And so on...
---- ----
/ \ / \
/ \ / \
--------------- ---------------- ---
R0:
--- ---- ---
\ / \ /
\ / \ /
---------------- ----------------
Simulation
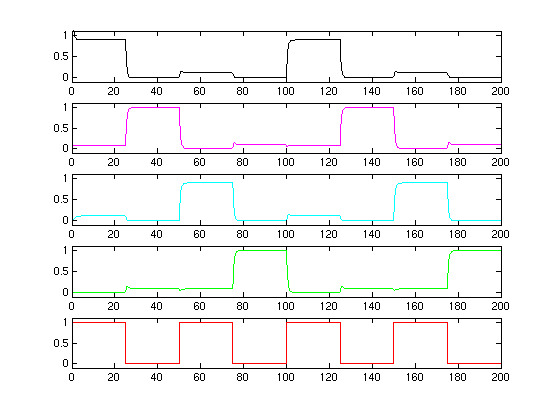
We did some early matlab simulations.
A first plot (although kind of overly optimistic regarding the parameter setting options) are showed here:The bottom plot is the input signal S. The four plot above are the concentration of the R[1,2,3,4]. As one can see, the cascade is clear and stable!
Challenges
- The combination of promoters, repressing and activating substances will be quite a challenge. They should change the state rather quick. Therefore most of the substances have to be expressed and decomposed quickly. But on the other hand the repression at certain points should stay long enough to ensure that there is only one state the system can fall in.
- I think the repression by the input signal S will affect the expression of R2 and R0 quickly. But will the decreasing concentration of S have the same affect on R1 and R3 in comparable time? Or is a second input needed with the reverse effect of S?
REPORTER
This will probably be one or more colors to indicate the current state of the counter.
Discussions
We invite anyone to make comments and participate in discussions here
Assessment
- Modularity
- The project is divided in modules that can be implemented and tested independently.
- The counter module itself is also very symmetrical.
- Very powerful combinatorial behaviour: combining two counter units could track 16 states, and so on!
- Reuse: the project is based on parts that have already been developed, such as the oscillator, or toggle switches in the counter design.
- Usefulness
- Counters and divisors have all kinds of interesting applications.
- Similar technology could be used to implement more complex finite state machines.
- Feasibility
- We have 4 genes and 12 regulatory pathways in the counter part. That is obviously not trivial.
- But the design is highly symmetrical, which mean that some parts could be reused, and relatively few parameters need to be tuned.
- Besides, the triggering by hand part is quite feasible and could allow us some last minute face-saving type of maneuver. We could limit our contribution to that, and rename the project, A H. Sapiens Powered Oscillator for Bacteria.
- Coolness
- All in all, i think everybody would agree that the project is in fact extremely nerdy. However, in a technical school, nerdy is cool. Therefore, the project is extremely cool.
In all fairness, this project also have some weaknesses:
- The philosophical implications are unclear.
- A stochastic counter with fuzzy logic unit could possibly be cooler.
- The counter cannot toast bread.
Intersection with Quorum_Sensing_based projects
- Triggering by Hand could be implemented using a quorum sensing inducer.
- Reporting would need the same type of visual stuff. We have not thought much about it, have you?
- Maybe there would be a bit of overlap with the cell division sensing mentioned on your page. Could you perhaps elaborate?
Attic
Have a look at Oscillator_based_Attic for old toggle stuff.
Back to the ETH Zurich main page.
[http://lucky7.to/timex/ timex watch co]