ETH2006 and
From 2006.igem.org
(→Sensitivity analysis) |
(→Sensitivity analysis) |
||
| Line 64: | Line 64: | ||
* Insensitivity regarding <tt>d-T7Rd/l-T7Rd</tt>, which verifies our model since degredation and translation of the deficient (truncated) mRNA polymerase has indeed no influence on the system output | * Insensitivity regarding <tt>d-T7Rd/l-T7Rd</tt>, which verifies our model since degredation and translation of the deficient (truncated) mRNA polymerase has indeed no influence on the system output | ||
* Various parameters are insensitive if at least one input is 0. This is clear since sythesized products from both input strands are needed for output stimulation. | * Various parameters are insensitive if at least one input is 0. This is clear since sythesized products from both input strands are needed for output stimulation. | ||
| - | * | + | * Some parameters of the reporter protein synthesis are sensitive even if both inputs are 0. This arises from the fact that there is always a constitutive transcription part even if the promoter is not activated by T7R. And in fact, <tt>p-Out</tt> controling the constitutive portion has higher sensitivity if at least one input is 0. |
| - | * Relatively high sensitivity to changes in the input | + | * Relatively high sensitivity to changes in the input rate 2 leading to tRNA production. Since tRNA is very stable, producing more or less tRNA has direct impact. The other input is less sensitive since T7R RNA polymerase is produced which has a much shorter half life period. Changing parameters affecting T7R production thus has weaker influence on the equilibrium concentration<br/>''Unclear: '' Why can't we see this in the input/output surface plot? |
* ... | * ... | ||
Revision as of 17:47, 29 October 2006
Contents |
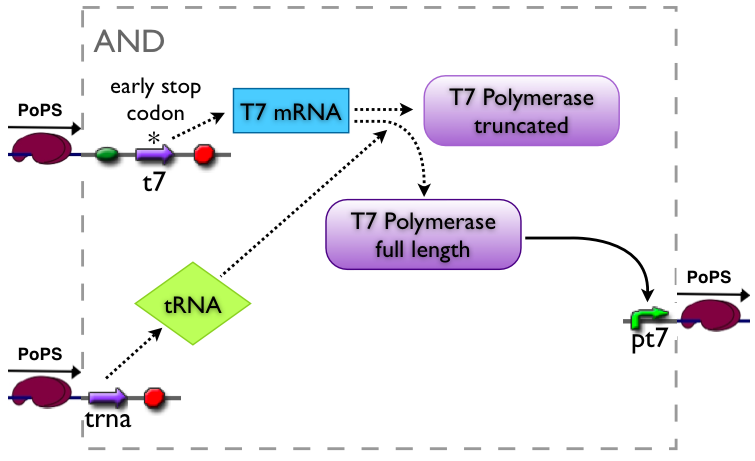
AND gate
The AND gate's PoPs output activity should be correlated to the PoPs input activity as shown in the picture:
input A ^
| L D H
| D D D
| L D L
+--------->
input B
output: High, Low, Dont care
Implementation alternatives
Part to be used in the prototype system: BBa_J34100
An AND gate produces an output only if both input signals are present. We want to implement such a “device” by using a suppressor tRNA. In our proposed model Signal 1 would lead to the transcription of the T7 RNA polymerase. However, an early stop codon was introduced into the coding sequence, leading to the expression of a truncated, non-functional protein. Signal 2, on the other hand, will lead to the expression of a suppressor tRNA. This tRNA recognizes the stop codon, preventing the end of translation and leading to incorporation of a glutamine into the nascent amino acid chain, so that translation can be completed. Consequently, a functional T7 RNA polymerase is present which will recognize its cognate promoter (T7 promoter) and therefore initiate expression of the downstream reporter gene (GFP).
Modeling
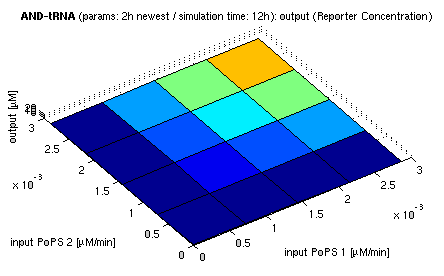
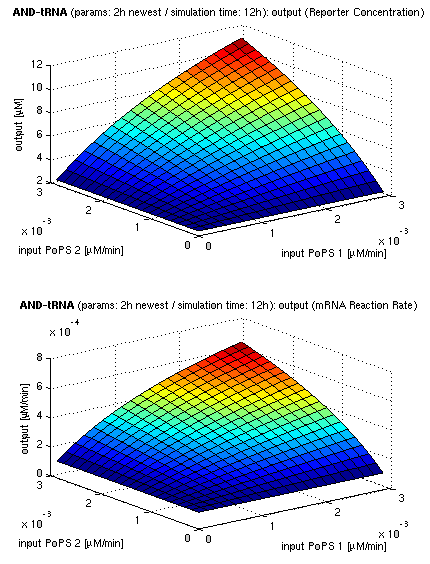
Current simulation results
| topside view, htProt=2h | 3d surface plot, htProt=2h |
| simulation results for AND gate, different protein halflife periods top / bottom: output (z axis) is mRNA rate (∼PoPS) / reporter concentration (x/y: inputs, z: output, htProt: protein halflife period) | |
For every plot, 25 (left) / 100 (right) ODE simulations were performed, simulating a time period of 12h. The simulations have been run with 2h halflife period for proteins. Complete parameter allocation can be found in the matlab scripts as well as the system of ordinary differential equations (ODEs).
The input rates (PoPS) have been chosen in the range of mRNA transcription rate, which was estimated according to the following assumptions:
- E.coli cytoplasm volume is approximately 6.7*10-16 l
- average number of mRNA molecules: 10
→ concentrationmRNA = 10/(6.7*10-16 * 6.022*1023) M = 0.0248 μM - at equilibrum, mRNA rate and degredation balance each other. Assuming half life period of 30min for mRNA, the result is
→ ratemRNA = concentrationmRNA * log(2)/30 μM/min = 5.7265e-04 μM/min
Amplifying/damping the input rates by small constant factors has influence on the qualitative outcome of the simulation.
- it is thus important to know how strong the input of the gate has to be.
- we can regulate this by choosing/designing the predecessor gate accordingly or
- by changing the ribosome binding sites to strengthen/weaken the input signal.
We accounted for this by adding restriction enzyme sites to the DNA, so that we can cut out current RBS to replace it by another of different strength.
The simulations show pretty much AND like behavior, but as mentioned, it is sensitive to the input strength. We want to find out about sensitivity more generally:
Sensitivity analysis
See sensitivity analysis for xor gate for description of the methods.
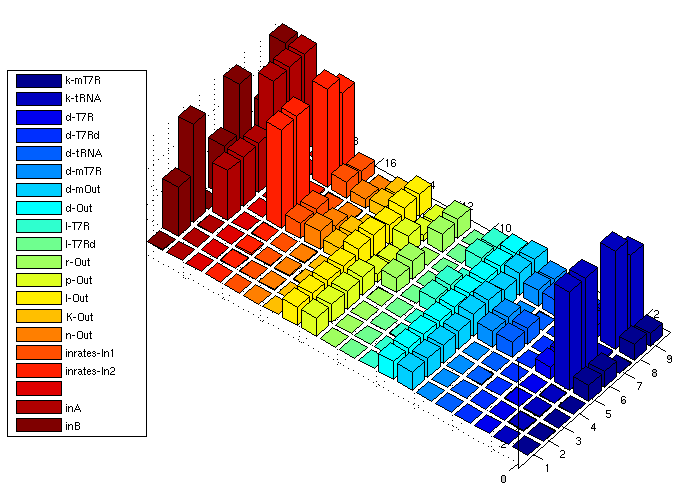
The following bar3 plot shows sensitivity matrices for different input combinations:
| AND: Sensitivity Analysis | |
| Normalized Sensitivity of the parameters for AND gate | |
The z axis shows the absolute normalized sensitivities, the other axes correspond to different input combinations (shorter axis) and the parameters. The left most series show inputs, not sensitivities (3x3 inputs, each [low, medium, high]). The unlabeled series is a placeholder for aesthetic purposes only. Detailed explanation of the parameter abbreviations can be found here. Parameters are postfixed with the state variable they belong to:
T7R : T7 RNA polymerase T7Rd : truncated (deficient) T7 RNA polymerase mT7R : mRNA for T7R tRNA : tRNA suppressing early stop codon Out : Reporter protein, here GFP mOut : mRNA for Out
The figure shows
- Insensitivity regarding d-T7Rd/l-T7Rd, which verifies our model since degredation and translation of the deficient (truncated) mRNA polymerase has indeed no influence on the system output
- Various parameters are insensitive if at least one input is 0. This is clear since sythesized products from both input strands are needed for output stimulation.
- Some parameters of the reporter protein synthesis are sensitive even if both inputs are 0. This arises from the fact that there is always a constitutive transcription part even if the promoter is not activated by T7R. And in fact, p-Out controling the constitutive portion has higher sensitivity if at least one input is 0.
- Relatively high sensitivity to changes in the input rate 2 leading to tRNA production. Since tRNA is very stable, producing more or less tRNA has direct impact. The other input is less sensitive since T7R RNA polymerase is produced which has a much shorter half life period. Changing parameters affecting T7R production thus has weaker influence on the equilibrium concentration
Unclear: Why can't we see this in the input/output surface plot? - ...
More (old) simulation results
simulation results / sensitivity analysis