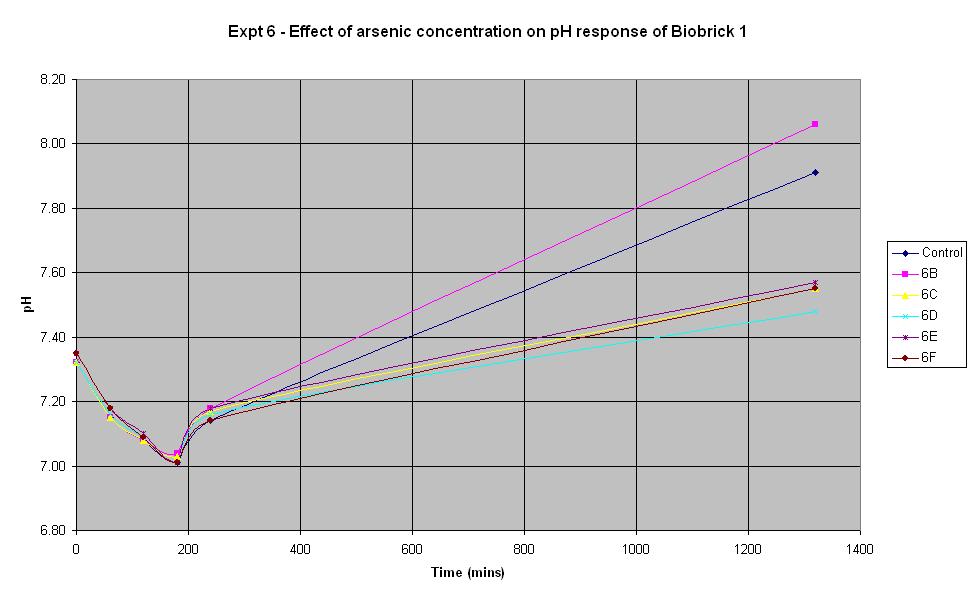
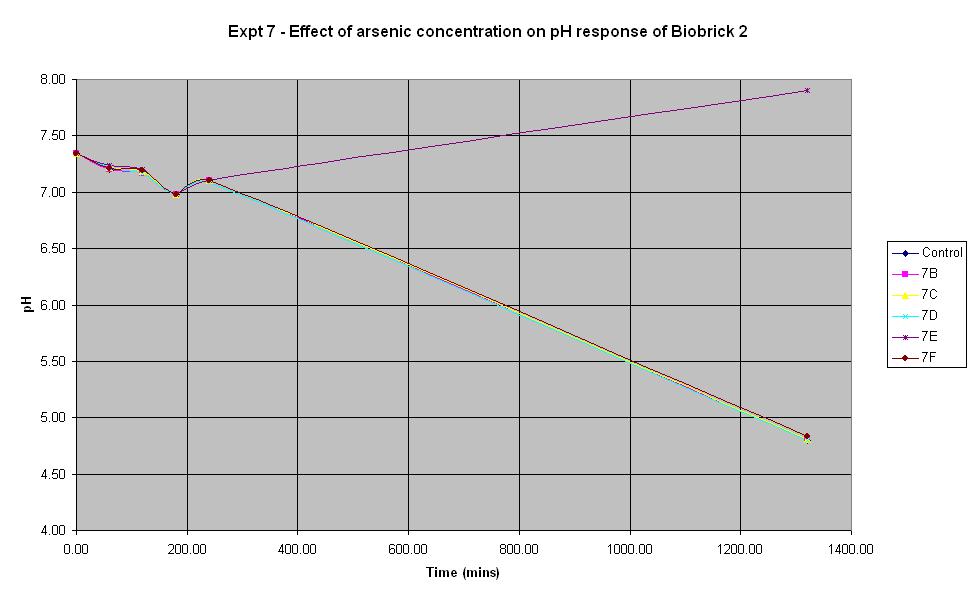
Biosensor Characterisation
From 2006.igem.org
| Line 36: | Line 36: | ||
Experiment 2 was carried out to find out what difference the volume of sterile water added to the solution makes to the rate of pH change. | Experiment 2 was carried out to find out what difference the volume of sterile water added to the solution makes to the rate of pH change. | ||
| + | |||
{| border="1" | {| border="1" | ||
| Line 62: | Line 63: | ||
|} | |} | ||
| + | It can be seen that all the dilutions behave in roughly the same way, especially when the error in pH of | ||
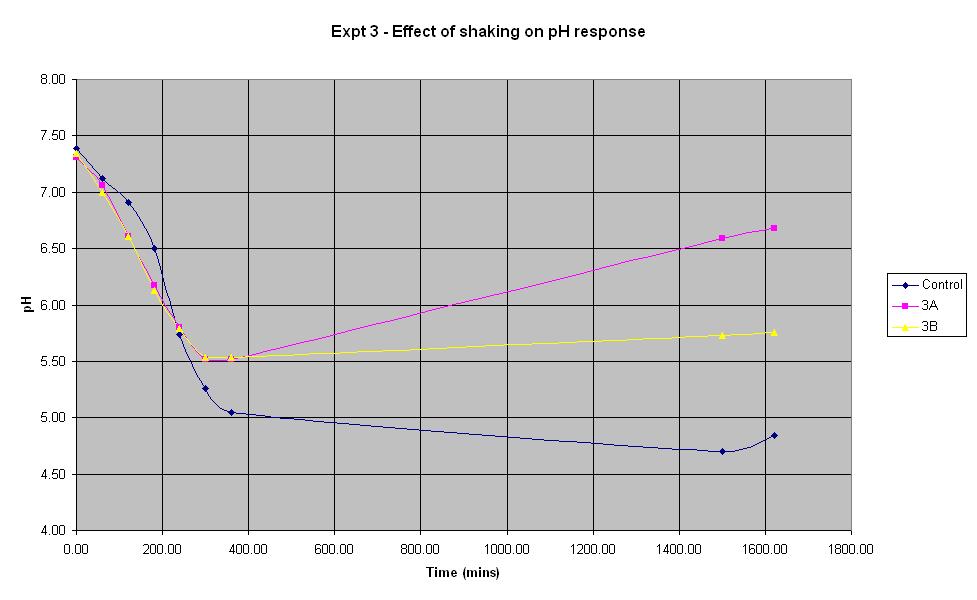
[[Image:Expt_3_graph.JPG | Experiment 3 Graph | 800 px]] | [[Image:Expt_3_graph.JPG | Experiment 3 Graph | 800 px]] | ||
Revision as of 11:13, 11 August 2006
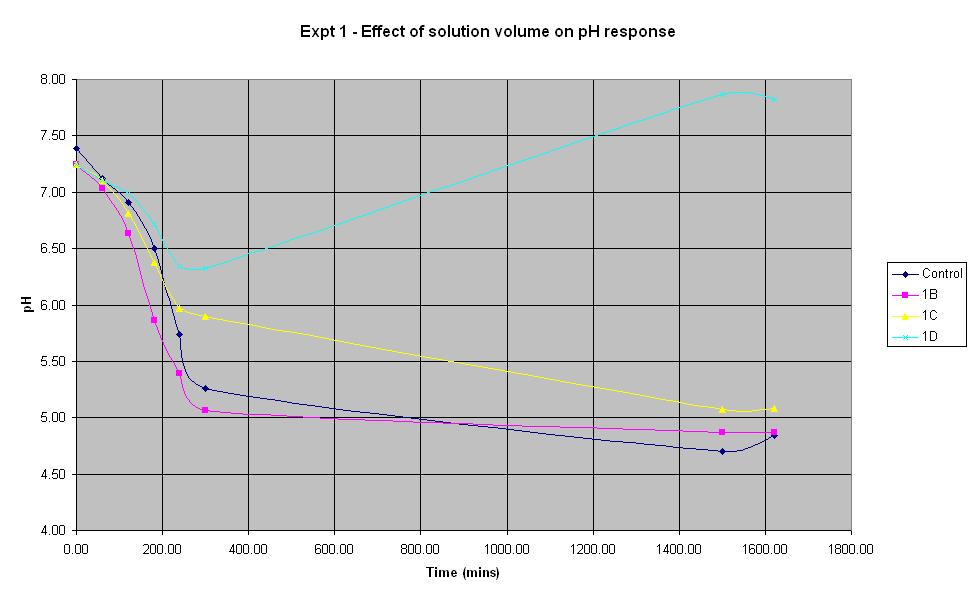
Experiment 1 was carried out using IPTG responsive E-Coli with LacZ to find out what difference the total solution volume makes to the rate of pH change.
| Experiment: | Control | 1B | 1C | 1D |
| LB (ml): | 50 | 25 | 10 | 5 |
| Culture (ml): | 5 | 3.125 | 1.25 | 0.625 |
| Lactose (ml): | 1.25 | 0.781 | 0.313 | 0.156 |
| Ampicillin (μl): | 10 | 6.25 | 2.5 | 1.25 |
| IPTG (μl): | 10 | 6.25 | 2.5 | 1.25 |
| Total volume (ml): | 56.27 | 28.919 | 11.568 | 5.784 |
The solution with total volume 28.1375 ml (1B) produced the fastest initial rate of pH decrease and reached approximately the same final pH as the control (pH 4.84).
The smallest solution volume changed pH at the slowest rate and after an initial decrease in pH, it then increased to 7.87 overnight.
Therefore, in the optimum order, 1B > Control > 1C > 1D
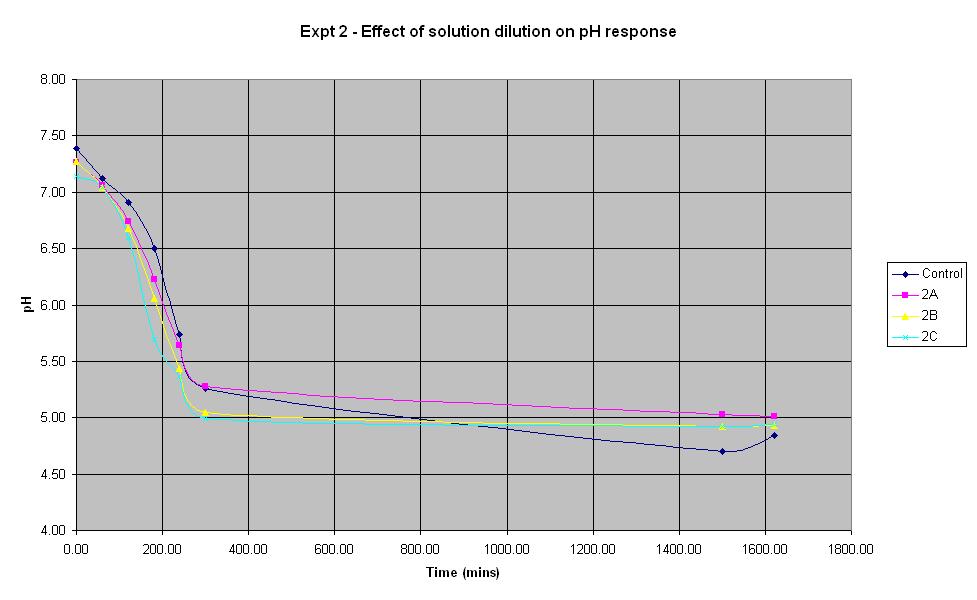
Experiment 2 was carried out to find out what difference the volume of sterile water added to the solution makes to the rate of pH change.
| Experiment: | Control | 2A | 2B | 2C | |
| LB (ml): | 50 | 50 | 50 | 50 | |
| Culture (ml): | 5 | 5 | 5 | 5 | |
| Lactose (ml): | 1.25 | 1.25 | 1.25 | 1.25 | |
| Ampicillin (μl): | 10 | 10 | 10 | 10 | |
| IPTG (μl): | 10 | 10 | 10 | 10 | |
| Sterile water (ml): | 0 | 50 | 25 | 10 | |
| Total volume (ml): | 56.27 | 106.27 | 81.27 | 66.27 |
It can be seen that all the dilutions behave in roughly the same way, especially when the error in pH of
[http://2006.igem.org/University_of_Edinburgh_2006 Main page]