Biosensor Characterisation
From 2006.igem.org
| Line 146: | Line 146: | ||
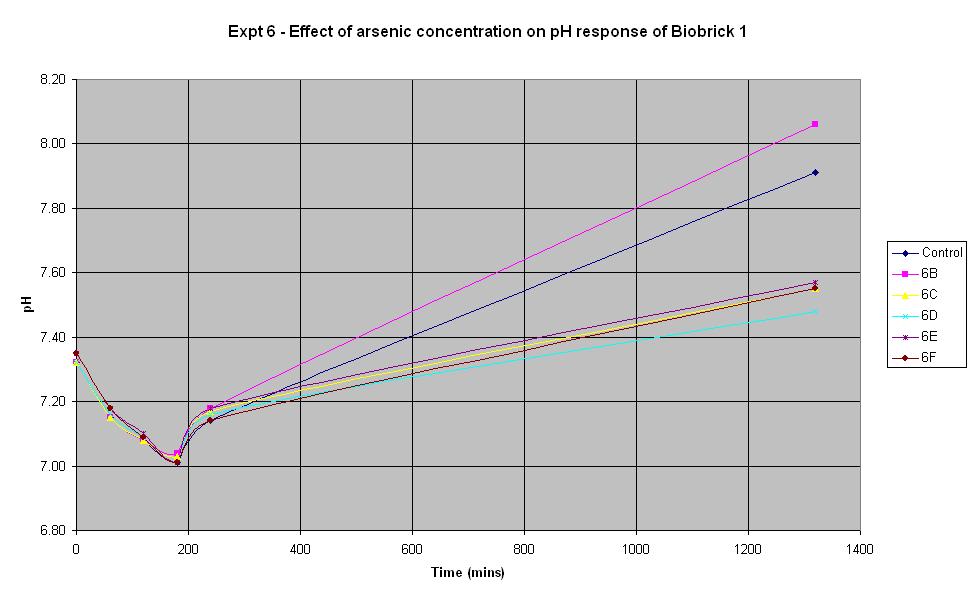
'''Experiment 6 - Effect of arsenic concentration on rate of pH change of arsenic responsive E-Coli (Biobrick construct 1)''' | '''Experiment 6 - Effect of arsenic concentration on rate of pH change of arsenic responsive E-Coli (Biobrick construct 1)''' | ||
| - | Experiment 6 was carried out using Biobrick construct 1 which has an arsenic promoter coupled to a LacZ. Different concentrations of arsenic were added to the solution to see which concentration produced a response. | + | Experiment 6 was carried out using Biobrick construct 1 which has an arsenic promoter coupled to a LacZ. Different concentrations of arsenic were added to the solution to see which concentration produced a response. |
| + | |||
| + | {| border="1" | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | !width="100"| | ||
| + | |- | ||
| + | | '''Experiment:''' || '''Control''' || '''6B''' || '''6C''' || '''6D''' || '''6E''' || '''6F''' | ||
| + | |- | ||
| + | | '''Arsenic (ppb):''' || 0 || 5 || 10 || 20 || 50 || 500 | ||
| + | |} | ||
[[Image:Expt_6_graph.JPG | Experiment 6 Graph | 800 px]] | [[Image:Expt_6_graph.JPG | Experiment 6 Graph | 800 px]] | ||
| Line 174: | Line 188: | ||
|} | |} | ||
| + | The results above show that the concentration of arsenic present does not affect the response of BioBrick construct 1 under the conditions shown in the table. | ||
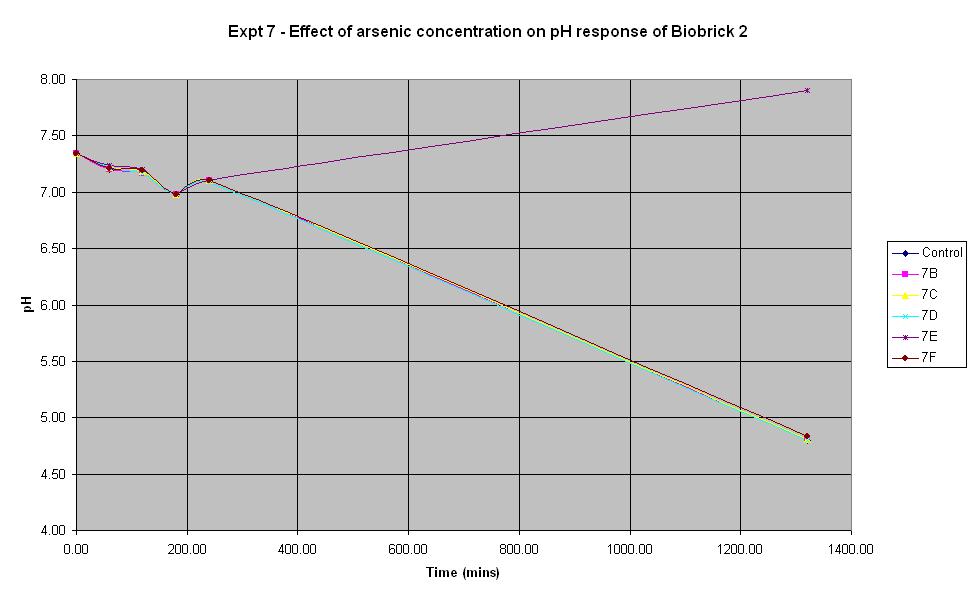
[[Image:Expt_7_Graph.JPG | Experiment 7 Graph | 800 px]] | [[Image:Expt_7_Graph.JPG | Experiment 7 Graph | 800 px]] | ||
Revision as of 13:47, 14 August 2006
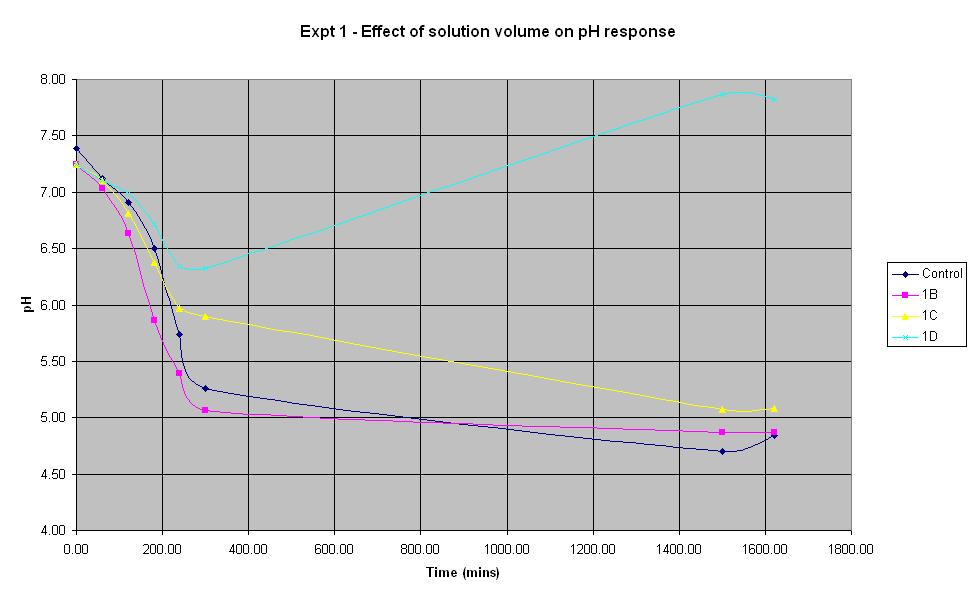
Experiment 1 - Effect of solution volume on rate of pH response
Experiment 1 was carried out using IPTG responsive E-Coli with LacZ to find out what difference the total solution volume makes to the rate of pH change.
| Experiment: | Control | 1B | 1C | 1D |
| Solution volume c.f control: | 100% | 50% | 20% | 10% |
| Experiment: | Control | 1B | 1C | 1D |
| LB (ml): | 50 | 25 | 10 | 5 |
| Culture (ml): | 5 | 3.125 | 1.25 | 0.625 |
| Lactose (ml): | 1.25 | 0.781 | 0.313 | 0.156 |
| Ampicillin (μl): | 10 | 6.25 | 2.5 | 1.25 |
| IPTG (μl): | 10 | 6.25 | 2.5 | 1.25 |
| Total volume (ml): | 56.27 | 28.919 | 11.568 | 5.784 |
The solution with total volume 28.1375 ml (1B) produced the fastest initial rate of pH decrease and reached approximately the same final pH as the control (pH 4.84).
The smallest solution volume changed pH at the slowest rate and after an initial decrease in pH, it then increased to 7.87 overnight.
Therefore, in the optimum order, 1B > Control > 1C > 1D
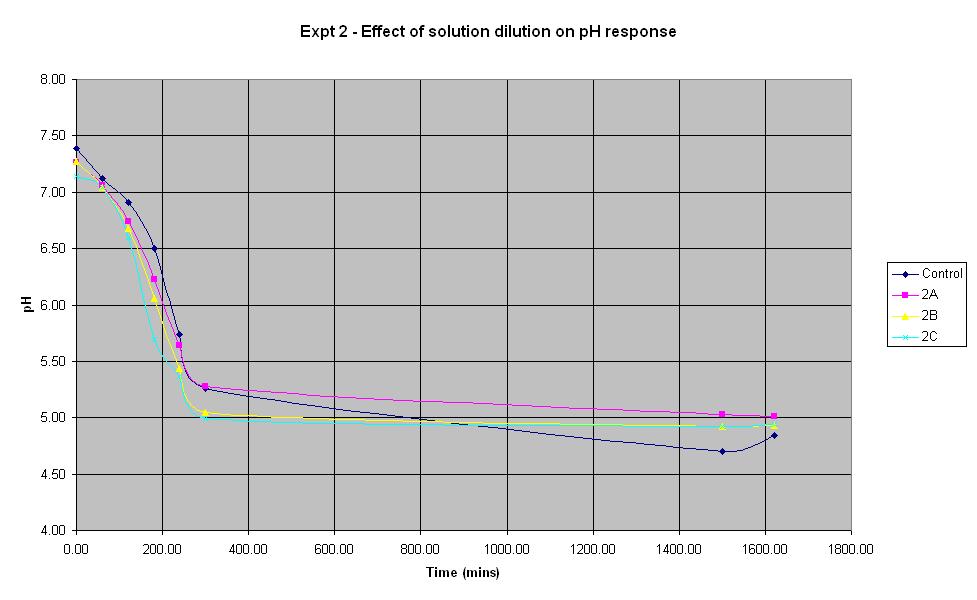
Experiment 2 - Effect of solution dilution on rate of pH response
Experiment 2 was carried out using IPTG responsive E-Coli with LacZ to find out what difference the volume of sterile water added to the solution makes to the rate of pH change.
| Experiment: | Control | 2A | 2B | 2C |
| Sterile water (ml): | 0 | 50 | 25 | 10 |
| Experiment: | Control | 2A | 2B | 2C |
| LB (ml): | 50 | 50 | 50 | 50 |
| Culture (ml): | 5 | 5 | 5 | 5 |
| Lactose (ml): | 1.25 | 1.25 | 1.25 | 1.25 |
| Ampicillin (μl): | 10 | 10 | 10 | 10 |
| IPTG (μl): | 10 | 10 | 10 | 10 |
| Sterile water (ml): | 0 | 50 | 25 | 10 |
| Total volume (ml): | 56.27 | 106.27 | 81.27 | 66.27 |
It can be seen that all the dilutions behave in roughly the same way, especially when the error in pH of +/- 0.2 is taken into account. The aim of this experiment was to find out whether it is feasible to dilute the standard culture solution by 50%. The pH of solution 2A which was diluted by 50% decreased at a rate very similar to that of the control solution, therefore it will be feasible to use this dilution in the final biosensor.
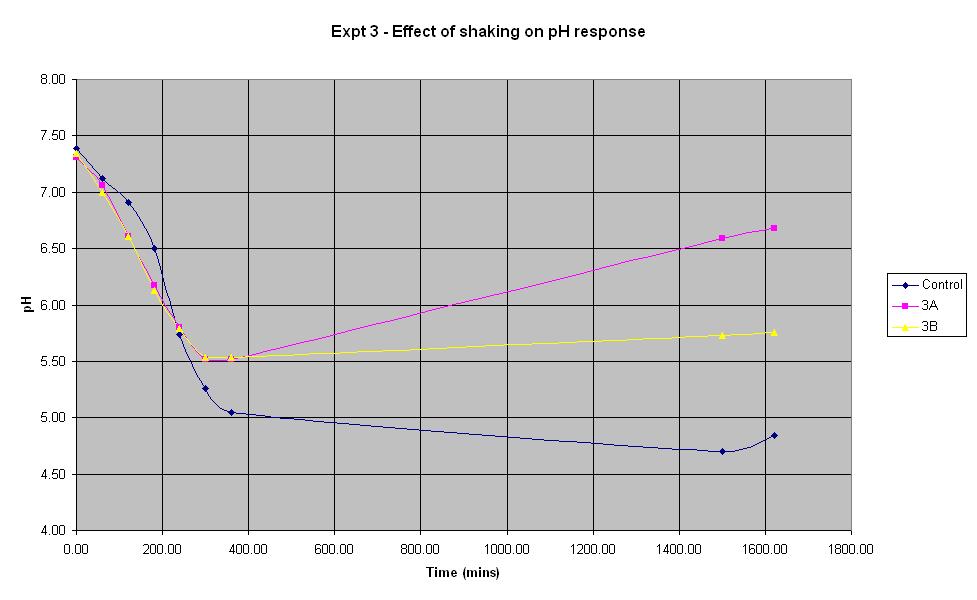
Experiment 3 - Effect of shaking on rate of pH response
Experiment 3 was carried out using IPTG responsive E-Coli with LacZ to find out what effect shaking has on the rate of pH change.
| Experiment: | Control | 3A | 3B |
| Shaken: | Yes | No | No |
The composition of solutions 3A and 3B is the same as that of the control (which is the identical to the control composition used in experiment 1). The only difference in the experiment is that neither solution 3A or 3B is shaken whereas the control is.
It can be seen that for the first 4 hours, the pH of the unshaken cultures decreases at a faster rate than that of the shaken control culture. However, the pH of the unshaken cultures stops falling after approximately 5 hours and begins to increase again. This shows that for the initial experimental period, it is not necessary to shake the biosensor but following the first 5 hours (approximately), shaking becomes more important.
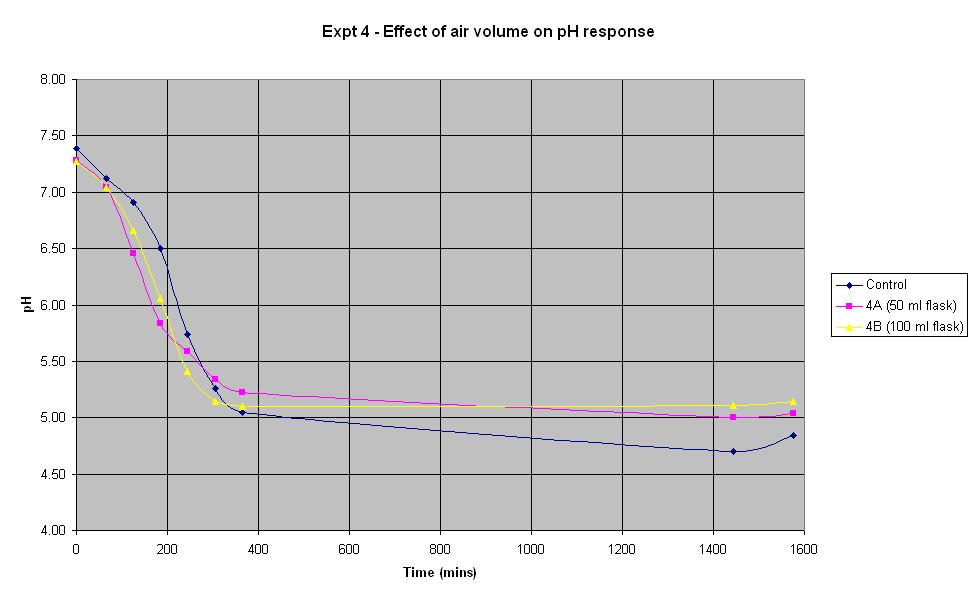
Experiment 4 - Effect of air volume on rate of pH response
Experiment 4 was conducted using different conical flask sizes to find out what effect the volume of air present has on the rate of pH change of IPTG responsive E-Coli with LacZ.
| Experiment: | Control | 4A | 4B |
| Conical flask size (ml): | 250 | 50 | 100 |
The composition and volume of 4A, 4B and the control solution is that used for the control in Experiment 1 above. The only difference in the experiment is the conical flask volume. The control is a 250 ml flask, 4A is a 50 ml flask and 4B is a 100 ml flask.
In the first 4 hours, the flask containing the smallest volume of air responded at the fastest rate. After about 5 hours, both 4A and 4B seemed to plateau out at a final pH just above 5 whilst the control continued to decrease slightly. However, if an error in pH of +/- 0.2 is considered, it can be seen there is not much difference in the rate of response between the flasks containing different air volumes.
Experiment 5 - Effect of freezing on pH universal indicator
This experiment was planned but not actually carried out as it became apparent that it would be better to freeze-dry the bacteria and nutrients rather than freezing them at -80 °C.
Experiment 6 - Effect of arsenic concentration on rate of pH change of arsenic responsive E-Coli (Biobrick construct 1)
Experiment 6 was carried out using Biobrick construct 1 which has an arsenic promoter coupled to a LacZ. Different concentrations of arsenic were added to the solution to see which concentration produced a response.
| Experiment: | Control | 6B | 6C | 6D | 6E | 6F |
| Arsenic (ppb): | 0 | 5 | 10 | 20 | 50 | 500 |
| Experiment: | Control | 6B | 6C | 6D | 6E | 6F |
| LB (ml): | 50 | 50 | 50 | 50 | 50 | 50 |
| Culture (ml): | 5 | 5 | 5 | 5 | 5 | 5 |
| Lactose (ml): | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| Ampicillin (μl): | 10 | 10 | 10 | 10 | 10 | 10 |
| Arsenic (μl): | 0 | 3.3 | 6.6 | 13.2 | 33 | 330 |
| Total volume (ml): | 56.273 | 56.274 | 56.277 | 56.283 | 56.303 | 56.600 |
The results above show that the concentration of arsenic present does not affect the response of BioBrick construct 1 under the conditions shown in the table.
[http://2006.igem.org/University_of_Edinburgh_2006 Main page]